Abstract
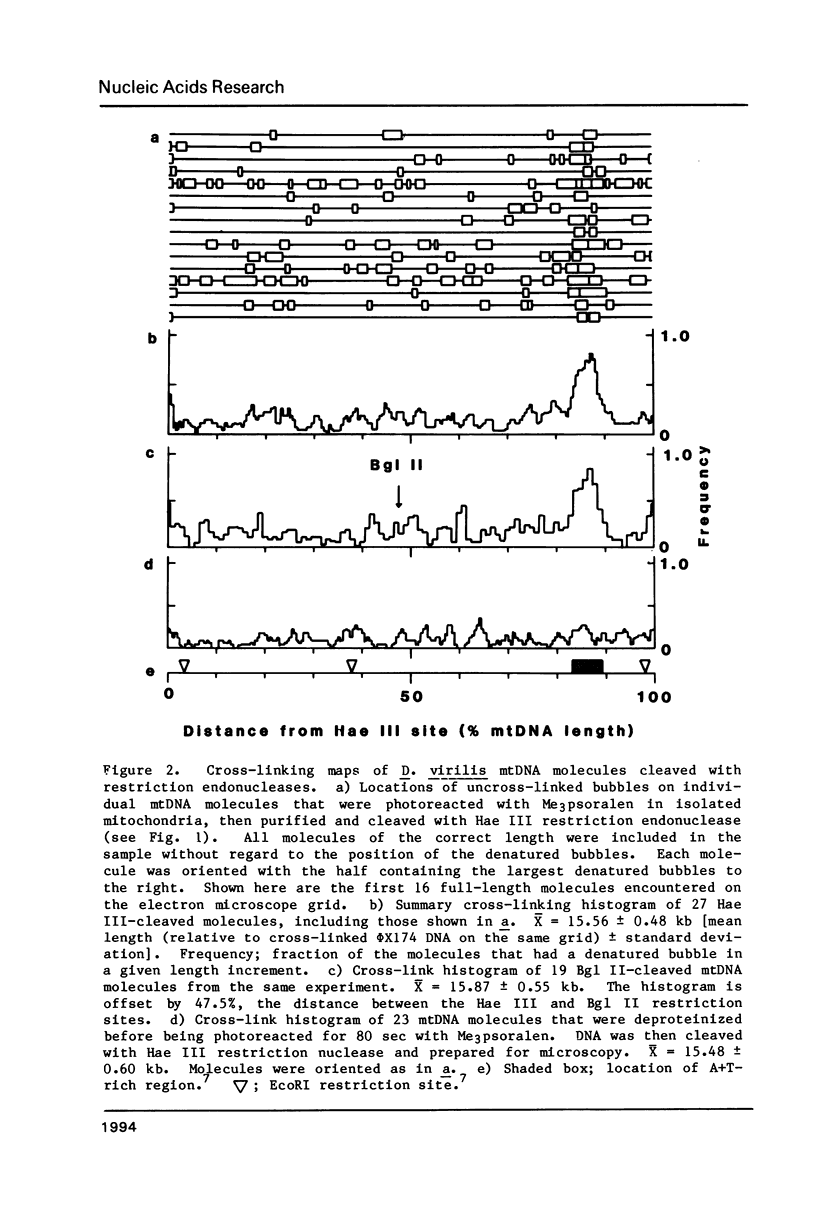
The location of proteins on the mitochondrial DNA (mtDNA) of Drosophila virilis was investigated by Me3 psoralen photoreaction of mitochondria isolated from embryos. After photoreaction the mtDNA was purified and the pattern of DNA cross-linking was determined by electron microscopy of the DNA under totally denaturing conditions. The transcribed regions of the mtDNA molecule contained some uncross-linked regions, but such regions were infrequent and randomly distributed. In contrast, the A + T-rich region around the origin of replication of the mtDNA was usually protected from psoralen cross-linking. The data were best fit by two protected sites, each approximately 400 base pairs, compared to the four 400 base pair sites observed in the equivalent region of D. melanogaster mtDNA [Potter et al. (1980) Proc. Nat. Acad. Sci. USA 77, 4118-4122]. Thus this region of the mtDNA appears to be involved in a DNA-protein structure that is highly conserved even though the DNA sequence has diverged rapidly relative to protein-coding sequences.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albring M., Griffith J., Attardi G. Association of a protein structure of probable membrane derivation with HeLa cell mitochondrial DNA near its origin of replication. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1348–1352. doi: 10.1073/pnas.74.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Cech T., Potter D., Pardue M. L. Electron microscopy of DNA cross-linked with trimethylpsoralen: a probe for chromatin structure. Biochemistry. 1977 Nov 29;16(24):5313–5321. doi: 10.1021/bi00643a024. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Goddard J. M., Martin S. C., Fauron C. M., Wolstenholme D. R. Drosophila mitochondrial DNA: a novel gene order. Nucleic Acids Res. 1982 Nov 11;10(21):6619–6637. doi: 10.1093/nar/10.21.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G. E., MacIntyre R. J. Microcomplement fixation studies on the evolution of alpha-glycerophosphate dehydrogenase within the genus Drosophila. Proc Natl Acad Sci U S A. 1977 Feb;74(2):684–688. doi: 10.1073/pnas.74.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco L., Attardi G. In situ photochemical crosslinking of HeLa cell mitochondrial DNA by a psoralen derivative reveals a protected region near the origin of replication. Nucleic Acids Res. 1981 Nov 25;9(22):6017–6030. doi: 10.1093/nar/9.22.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Extensive diversity among Drosophila species with respect to nucleotide sequences within the adenine + thymine-rich region of mitochondrial DNA molecules. Nucleic Acids Res. 1980 Jun 11;8(11):2439–2452. doi: 10.1093/nar/8.11.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Intraspecific diversity of nucleotide sequences within the adenine + thymine-rich region of mitochondrial DNA molecules of Drosophila mauritiana, Drosophila melanogaster and Drosophila simulans. Nucleic Acids Res. 1980 Nov 25;8(22):5391–5410. doi: 10.1093/nar/8.22.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3886–3890. doi: 10.1073/pnas.75.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from the genus Drosophila. Nucleic Acids Res. 1980 Feb 25;8(4):741–757. [PMC free article] [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Merten S. H., Pardue M. L. Mitochondrial DNA in Drosophila. An analysis of genome organization and transcription in Drosophila melanogaster and Drosophila virilis. J Mol Biol. 1981 Nov 25;153(1):1–21. doi: 10.1016/0022-2836(81)90523-4. [DOI] [PubMed] [Google Scholar]
- Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980 Nov;22(2 Pt 2):393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- Pardue M. L. Repeated DNA sequences in the chromosomes of higher organisms. Genetics. 1975 Jun;79 (Suppl):159–170. [PubMed] [Google Scholar]
- Potter D. A., Fostel J. M., Berninger M., Pardue M. L., Cech T. R. DNA-protein interactions in the Drosophila melanogaster mitochondrial genome as deduced from trimethylpsoralen crosslinking patterns. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4118–4122. doi: 10.1073/pnas.77.7.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Langley C. H. Electron microscope heteroduplex study of Drosophila mitochondrial DNAs: evolution of A+T-rich region. Plasmid. 1979 Jan;2(1):69–78. doi: 10.1016/0147-619x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]



