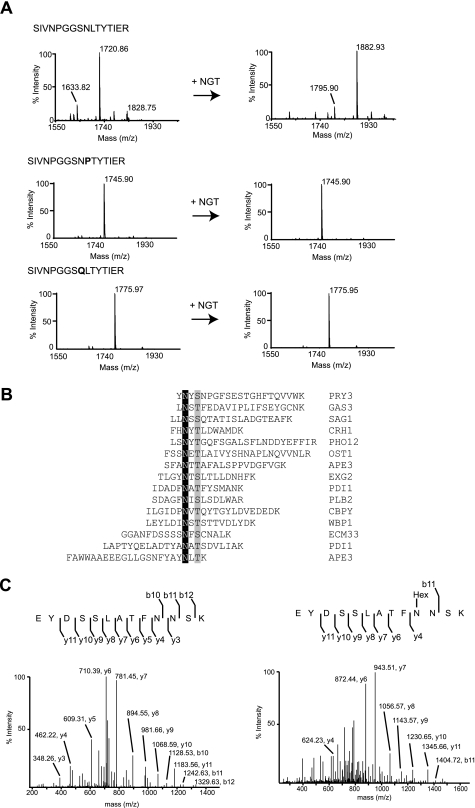
FIGURE 4.
Overlapping substrate specificity of NGT and OST. A, MALDI-MS analysis of the glycosylation products using model peptides in the presence of UDP-glucose without (left panels) or with (right panels) NGT. Upper panels, spectrum of the SIVNPGGSNLTYTIER peptide (calculated molecular mass, 1720.89 Da); middle panels, spectrum of the acetyl-SIVNPGGSNPTYTIER-amide peptide (calculated molecular mass, 1745.87 Da); lower panels, spectrum of the acetyl-SIVNPGGSQLTYTIER-amide peptide (calculated molecular mass, 1775.97 Da). B, list of synthetic peptides that are glycosylated by NGT. The modified asparagine residues are highlighted in black. The amino acids in position +2 are shown in gray. The peptide sequences derive from the yeast glycoproteins identified by the UniProt entry names indicated on the right. C, LC-electrospray ionization-MS/MS analysis of the tryptic products of AcrA. Left panel, the spectrum from fragmentation of the doubly charged precursor ion at m/z 738.34 corresponds to the peptide EYDSSLATFNNSK. Right panel, the spectrum from fragmentation of the doubly charged ion at m/z 819.36 matches the glycopeptide EYDSSLATFN(Glc)NSK.