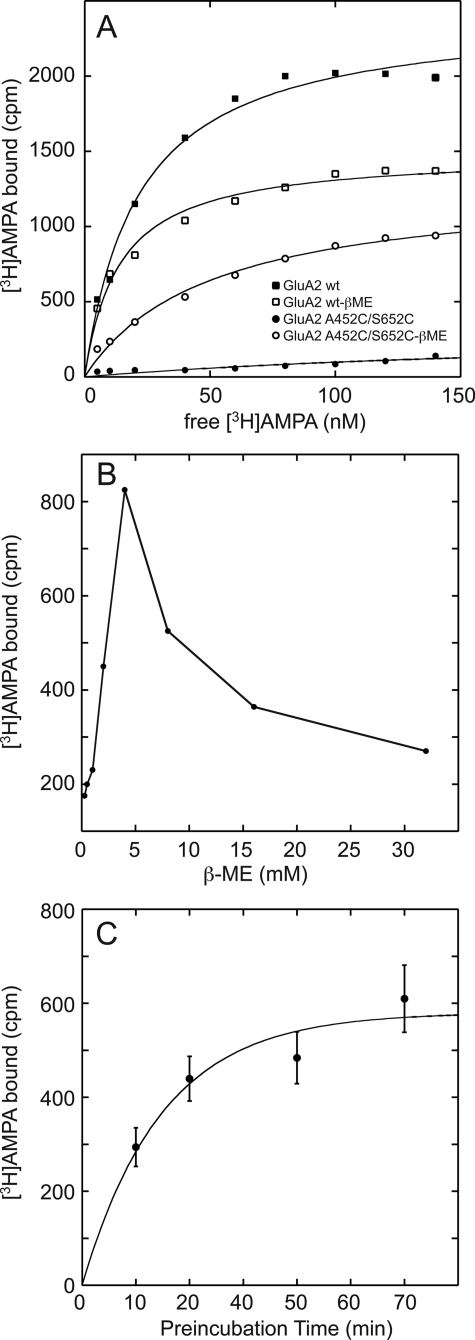
FIGURE 3.
A, binding of [3H]AMPA to both wild type and the A452C/S652C mutant GluA2 LBD. For the wild type, a decrease in binding was observed in the presence of 5 mm β-metcaptoethanol (apparent decrease in Bmax). In the case of the mutant, binding is only observed when the disulfide bond is reduced by β-metcaptoethanol (β-ME). The KD for binding of [3H]AMPA to the reduced form of the mutant is 2-fold higher than that observed for wild type. B, treatment with β-metcaptoethanol increases binding of the A452C/S652C mutant up to 5 mm, and above that concentration, binding decreases. This may be due to the initial selective reduction of the A452C/S652C followed by the reduction of the Cys-722/Cys-773 disulfide bond. C, the time course for reduction of the disulfide bond shows that it is complete within 60 min in the presence of 5 mm β-metcaptoethanol.