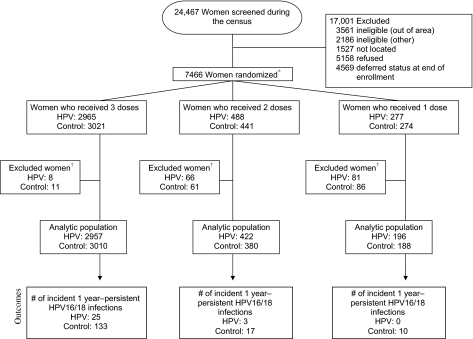
Figure 1.
CONSORT diagram of women in the Costa Rica Vaccine Trial. The primary aim of the trial was to evaluate the efficacy of a three-dose regimen of the Cervarix vaccine to prevent persistent type-specific infection with HPV16 or HPV18 and the subsequent development of HPV-associated precancerous lesions. Although 7466 women were randomized to receive three doses of either Cervarix or control vaccine, approximately 20% received fewer than three doses of Cervarix or control vaccine mostly because of pregnancy and referral to colposcopy. Thus, we were able to investigate the protection afforded by two and one dose(s) of the HPV vaccine because the cost and logistical difficulties of the standard three-dose vaccine regimen compromises implementation. Asterisk indicates that four women received discordant vaccines (one woman was enrolled twice and received three doses of each vaccine and three women received two doses of one vaccine and one dose of the other vaccine). For the purpose of this analysis, the control dosing was ignored and they were categorized based on the number of HPV vaccines they received. Dagger indicates that women who were both HPV16 and HPV18 DNA positive at enrollment were excluded, as were women with no follow-up visits post-enrollment.