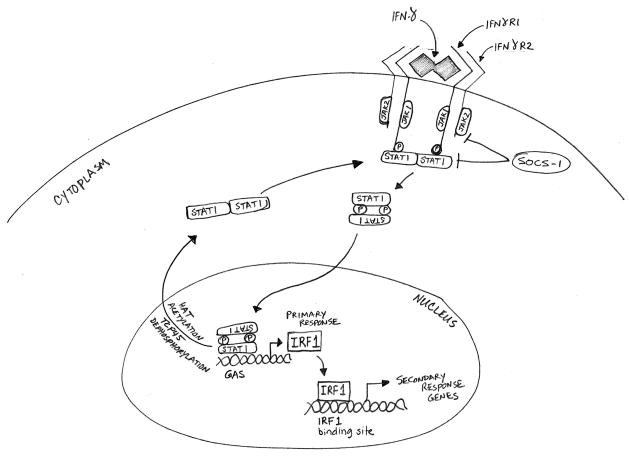
Figure 1.
The canonical IFN-γ/JAK/STAT pathway. Binding of IFN-γ dimers to the extracellular domain of the IFNγR1 receptor subunit leads to engagement of the IFNγR2 subunit, which causes JAK1 and JAK2 to cross-phosphorylate each other and the receptor subunits. The parallel STAT1 homodimers are then recruited to the receptors, and their phosphorylation converts the homodimers into an antiparallel configuration. The reoriented STAT1 homodimers translocate to the nucleus, where they bind to gamma activated sequence (GAS) sites on the primary response genes including IRF1. IRF1 subsequently activates a large number of secondary response genes, which carry out a range of immunomodulatory functions. The SOCS proteins serve as the major negative regulators of the IFN-γ pathway by inhibiting the phosphorylation of JAKs and STAT1. Dephosphorylation and acetylation of STAT1 homodimers revert them to parallel configuration and causes their exit from the nucleus.