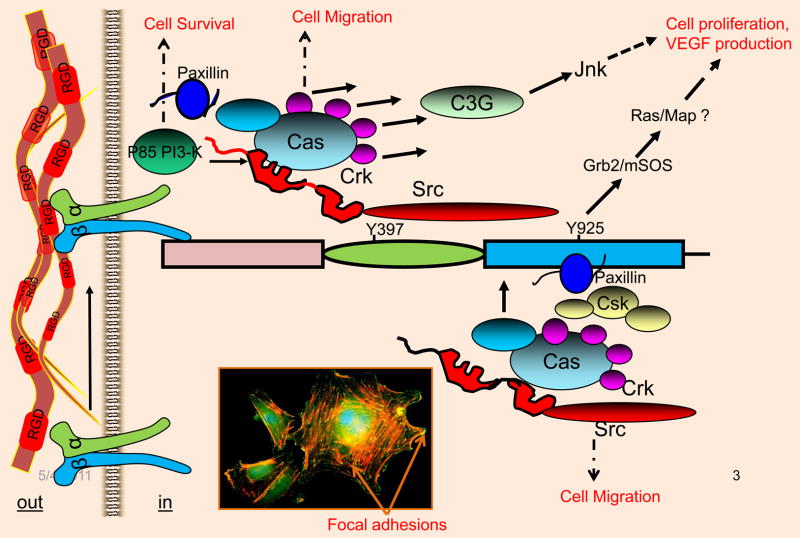
Fig. 3.
Interaction of FAK with signaling and cytoskeletal proteins. In response to integrin ligation, FAK is autophosphorylated at the Y397 residue. Phosphorylated Y397 recruits Src via its SH2 domain. Recruited Src tyrosine kinase then transphosphorylates the Y929 residue, which is the binding site for the Grb2/mSOS complex. The N-terminal FERM domain can also be activated by growth factor receptor systems, including the VEGF/VEGFR2 system. This event can further induce tyrosine phosphorylation of other sites, which creates additional docking sites for signaling and cytoskeletal proteins, including p130Cas, paxillin, PI-3-kinase and Crk. Inset: Endothelial cells plated onto fibronectin coated glass slide was immunostained with anti-FAK antibody (green) and TRITC-Phalloidin (red); yellow structures indicated by arrows show colocalization at Focal adhesion sites.