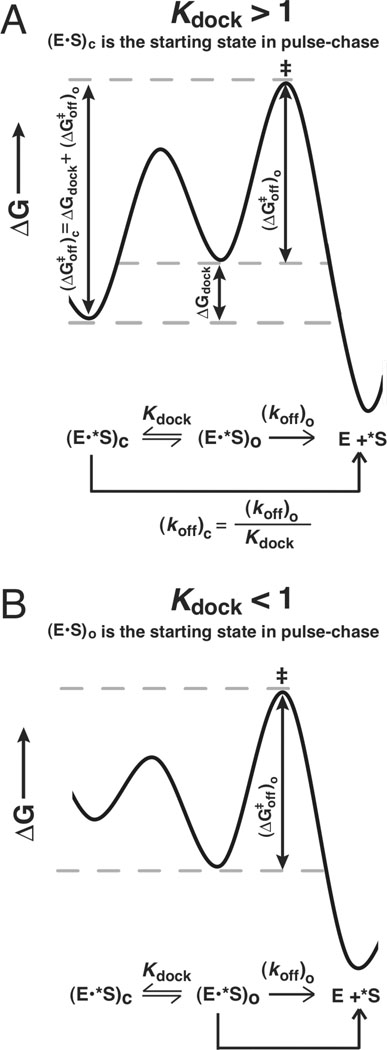
Figure 2.
The dissociation constants for the oligonucleotide substrate from the open and closed complexes can be used to determine the equilibrium constant for docking (Kdock). A. The free energy diagram for a closed complex substrate, which allows measurement of . ΔGdock can be obtained from the difference between and [i.e., ] B. The free energy diagram for an open complex substrate, which allows measurement of The values of ΔG and the corresponding dissociation rate and equilibrium constants can be interconverted via standard equations as described in Measurement of docking equilibria in Materials and Methods.