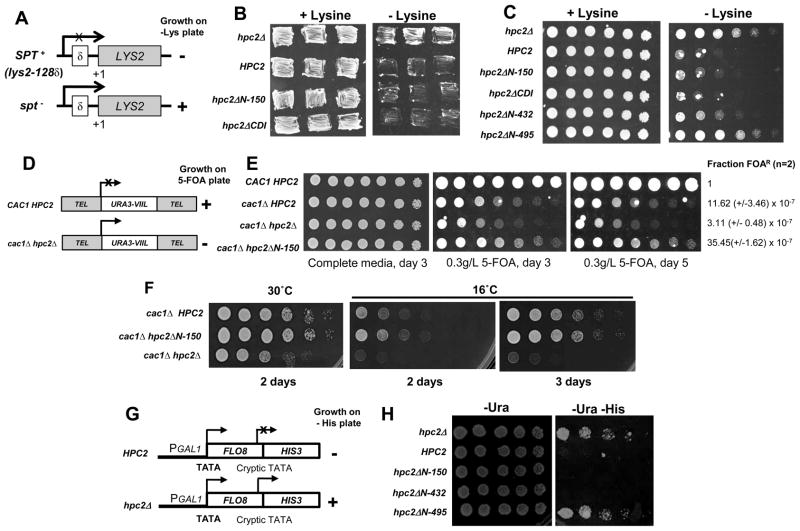
Figure 5. The hir/hpc dependent deregulation of the histone genes is not responsible for the other hir/hpc-associated phenotypes.
(A) Schematic of the Spt assay phenotype. Mutations in SPT gene (Spt−) suppresses the transcriptional defect of the LYS2 gene in the SPT+ strain caused by insertion of the Ty element. The Spt phenotype is measured by growth on media lacking lysine. (B) The Hpc2 mutant deleted of the CDI domain presents an SPT+ phenotype similar to Hpc2 WT. Cells of yeast strain YPP940 transformed with the indicated YIp:TRP1 plasmid were patched onto synthetic complete media with or without Lysine to assess the Spt phenotype. (C) The first 432 amino acids of Hpc2 are dispensable for the SPT+ phenotype. Fourfold serial dilutions of cells from the YPP940 strain transformed with the indicated YIp:TRP1 plasmid were spotted onto synthetic complete media with or without lysine plates and grown for 5 days at 23°C. (D) Schematic of the telomeric silencing loci (URA3-VIIL). The URA3 gene is inserted next to the chromosome VIIL telomere [58]. Under a wild-type background, URA3 is silenced and so cells cannot metabolize 5-FOA into a lethally toxic metabolite and thus will survive. However mutations affecting telomeric silencing leads to URA3 expression resulting into toxicity and cell death in presence of 5-FOA. (E) Telomeric silencing defects of the hir/hpcΔ mutant when combined to cac1Δ are not due to hir/hpc-dependent histone gene derepression. Threefold serial dilution of cells of the yeast strain YPP1200 (URA3-VIIL) transformed with the indicated YIp:TRP1 plasmid were spotted onto synthetic complete media with or without 5-FOA and grown at 30°C. As previously reported by Sharp and colleagues [47], the cac1Δ mutant alone displays a severe loss of telomeric silencing, which is further exacerbated upon deletion of HIR/HPC genes. Therefore, we had to decrease the amount of 5-FOA to reduce the toxicity of the cac1Δ mutant alone and to observe enhanced sensitivity due to the hir/hpc-dependent loss of telomeric silencing. The cac1Δ HPC2 and cac1Δ hpc2ΔN-150 have a reduced sensitivity to 5-FOA compare to the cac1Δ hpc2Δ double mutant as shown by their better growth. The fraction of viable cells resistant to FOA (FOAR) was normalized to the value obtained for the wild-type (CAC1 HPC2). The averages of data from two experiments carried out with duplicate plating are shown (F). The hpc2ΔN-150 mutant does not display any synthetic temperature sensitive growth defect when combined with cac1Δ mutant. Cells of strains used in (D) were spotted on YPD media and incubated at 30°C or at 16°C for the indicated number of days. (G) Schematic of the pGAL1::FLO8-HIS3 reporter gene (adapted from [19]). (F) hir/hpc-dependent histone gene derepression is not responsible for the HIR-dependent inhibition of transcription initiation from the cryptic promoter of the pGAL1::FLO8-HIS3 reporter gene. The yeast strain L1133 containing the pGAL1::FLO8::HIS3 reporter, described in [19], was transformed with the indicated YCp:URA3 plasmid. Fourfold serial dilution of cells were spotted onto SD -Ura or SD -Ura -His to assess for the derepression of the FLO8 cryptic promoter allowing expression of HIS3 and growth in absence of histidine. Similarly to (C), most of Hpc2 is dispensable for the HIR-mediated repression of the FLO8 cryptic promoter. Only further N-terminal truncation which removed the CDII domain (hpc2ΔN-495), necessary for Hpc2 to interact with the rest of the HIR complex, displays a growth on -His media similar to hpc2Δ showing a loss of repression of the cryptic promoter.