Abstract
Ethionamide (ETH), a second-line antituberculosis drug, is frequently used in treating childhood tuberculosis. Data supporting ETH dose recommendations in children are limited. The aim of this study was to determine the pharmacokinetic parameters for ETH in children on antituberculosis treatment including ETH. ETH serum levels were prospectively assessed in 31 children in 3 age groups (0 to 2 years, 2 to 6 years, and 6 to 12 years). Within each age group, half received rifampin (RMP). Following an oral dose of ETH (15 to 20 mg/kg of body weight), blood samples were collected at 0, 1, 2, 3, 4, and 6 h following 1 and 4 months of ETH therapy. The maximum serum concentration (Cmax), time to Cmax (Tmax), and area under the time-concentration curve from 0 to 6 h (AUC0–6) were calculated. Younger children were exposed to lower ETH concentrations than older children at the same mg/kg body weight dose. Age correlated significantly with the AUC after both 1 month (r = 0.50, P = 0.001) and 4 months (r = 0.63, P = 0.001) of therapy. There was no difference in the AUC or Cmax between children receiving concomitant treatment with RMP and those who did not. Time on treatment did not influence the pharmacokinetic parameters of ETH following 1 and 4 months of therapy. HIV infection was associated with lower ETH exposure. In conclusion, ETH at an oral dose of 15 to 20 mg/kg results in sufficient serum concentrations compared to current adult recommended levels in the majority of children across all age groups. ETH levels were influenced by young age and HIV status but were not affected by concomitant RMP treatment and duration of therapy.
INTRODUCTION
The thioamides, ethionamide (ETH) and prothionamide, are among the most frequently used second-line drugs for the management of pediatric tuberculosis (TB) and are active against several species of mycobacteria. They are oral drugs recommended for treatment of multidrug-resistant tuberculosis (MDR-TB) and also used to treat drug-susceptible tuberculous meningitis (TBM) and miliary disease due to good cerebrospinal fluid penetration (8, 19). Absorption of ETH from the intestinal tract is almost complete, and food and antacids appear to have little effect on this process (2). Protein binding is approximately 30% (9). ETH is distributed with ease throughout the body (28). In adults, peak plasma concentrations of ETH occur at approximately 2 h postdosing (2, 10, 29). For clinical purposes, the suggested serum levels for susceptible strains of Mycobacterium tuberculosis vary in the literature, as follows: 2.5 μg/ml or between 1 and 5 μg/ml (13, 14, 22). There is evidence that ETH has a bactericidal effect in higher doses (15). Higher doses are often poorly tolerated, limiting the dose in adults to 500 to 1,000 mg per day. A very small proportion of the drug is excreted unchanged, and the first step in thioamide metabolism in the liver is transformation to the active sulfoxide metabolites by monooxygenases (17). These monooxygenases have many properties in common with the cytochrome P450 system (CYP) and often have overlapping substrate specificities (17). Rifampin (RMP), commonly used with ETH, is a potent inducer of hepatic and intestinal cytochrome P450 enzymes and p-glycoprotein and activates the nuclear pregnane X receptor and the constitutive androstane receptor (20).
The optimal dose of ETH for children has not yet been established. While a total daily pediatric dose of 10 to 20 mg/kg of body weight has been suggested by the WHO and 15 to 20 mg/kg is the standard in South Africa, the resulting serum levels in children of different ages are largely unknown. Little is known regarding the accumulation of ETH during continuous therapy or whether drug interactions relevant to combination TB therapy in children lead to changes in ETH serum levels over time.
In drug-susceptible tuberculous meningitis or miliary disease, ETH is given in a combination regimen which includes RMP. The effects of coadministered drugs, such as RMP, on the concentrations of ETH are unknown.
We studied ETH serum levels in children of different age groups who were routinely on two different treatment regimens in a hospital setting at 1 and 4 months after initiation of routine antituberculosis treatment. Besides a single previous study of two children, this is to our knowledge the first evaluation of ETH pharmacokinetics in children (29).
MATERIALS AND METHODS
This study was conducted at Brooklyn Hospital for Chest Diseases (BHCD), Cape Town, South Africa. From September 2009 through May 2010, children aged 3 months to 13 years admitted to BHCD were eligible for enrollment. Children were divided in 3 age groups (3 months to 2 years, 2 to 5 years, and 6 to 13 years of age), with 10 children in each age stratum, of whom half routinely received RMP.
All parents or legal guardians gave written informed consent for their child's participation prior to enrollment. The study was approved by the Health Research Ethics Committee of the Faculty of Health Sciences of Stellenbosch University, South Africa, where ETH is licensed for use in children.
Diagnosis of tuberculosis.
The diagnosis of TB was based on a chest radiograph compatible with a diagnosis of pulmonary TB and/or clinical signs of extrapulmonary TB (e.g., peripheral lymph nodes, meningitis, abdominal TB) with supporting special investigations, including mycobacterial culture. Middlebrook 7H9 broth base (mycobacterial growth indicator tubes [MGIT]; Becton Dickinson, Sparks, MD) culture medium was used for primary isolation. The presence of M. tuberculosis was confirmed by PCR amplification. Phenotypic drug susceptibility testing was done using the Bactec 460TB system (Becton Dickinson, Sparks, MD), according to international criteria. Strain susceptibility was judged by comparing growth of organisms in drug- versus nondrug-containing media. Resistance was defined as 1% or more bacterial growth in the drug-containing media (24). A history of household TB contact and a positive Mantoux tuberculin skin test (TST), along with suggestive symptoms, were considered supportive evidence. When no culture was obtained, children were treated empirically according to the drug susceptibility result of isolates from the likely source case.
Treatment.
Children were included if ETH was part of their standard antituberculosis daily treatment and only if they could tolerate taking ETH as a single daily dose. South African Medicines Control Council-approved ETH 250-mg tablets (Sanofi Aventis, South Africa) were used.
All children received supplementary pyridoxine and multivitamin syrup. Trimethoprim-sulfamethoxazole was given to all HIV-infected children. Antiretroviral treatment consisted of two nucleoside reverse transcriptase inhibitors (lamivudine and stavudine) and either lopinavir-ritonavir (boosted with extra ritonavir if on RMP) in children younger than 3 years of age or efavirenz in children older than 3 years of age.
Pharmacokinetic investigation.
A routine recommended ETH dose of 15 to 20 mg per kg of body weight was calculated for each child, and ETH tablets were measured accordingly. ETH was administered by study personnel in the morning after an overnight fast (minimum of 4 h of fasting in younger children). In children who were <2 years of age and if necessary in other children, ETH tablets were crushed and suspended in 2 to 5 ml of water. For one child, all drugs were given via a gastrostomy tube. Children had breakfast only after the 1-hour blood sample was taken. In case of HIV infection, antiretroviral therapy was given with breakfast, while other treatment was given after the last blood sample was taken.
Blood samples were collected at 0, 1, 2, 3, 4, and 6 h following dosing and following 1 and 4 months of antituberculosis therapy.
Blood samples were collected in EDTA-containing sampling vials, centrifuged, frozen at −80°C, and stored. To 100 μl of sample, 300 μl of methanol, containing 1.0 μg/ml thiacetazone (Sigma, St. Louis, MO), was added. The supernatant was transferred into autosampler vials for analysis using a 5-μl injection volume.
Specimens were analyzed using a binary high-performance liquid chromatographer (HPLC) (Agilent series 1100 HPLC; Agilent Technologies, Waldbronn, Germany) equipped with an Agilent Zorbax analytical column (150 mm by 2.1 mm inside diameter [i.d.], 3.5-μm particle size). The column temperature was maintained at 40°C at a flow rate of 300 μl/min. The mobile phase A was water containing 0.1% formic acid (FA) (Fluka Chemie GmbH, Buchs, Switzerland), while phase B was methanol (E. Merck, Darmstadt, Germany) containing 0.1% FA. All solvents were of HPLC grade. The concentration of ETH was determined using an API 2000 tandem mass spectrometer (MS/MS; Applied Biosystems, MDS Sciex, Foster City, CA) equipped with an atmospheric turbulon ionization chamber. A single transition range for ETH of m/z 167.13/107.00 was used, while for the internal standard, a transition of m/z 237.12/119.96 was used. All samples and spiked standards were analyzed in duplicate. The within-day variation between the duplicates was less than 2%. The overall analytical precision over the entire duration of the study was not more than 3% over the entire calibration range. The day-to-day variation of standards and patient samples was in the range of 1.5 to 2.0%. The retention times of ETH and thiacetazone were 2.73 min and 7.0 min, respectively. The temperature of the nebulizer gas of the ionization chamber was set to be 400°C. Spiked calibrators in the range of 0.5 μg/ml to 15.0 μg/ml showed a linear relationship. Quality control samples were added to each sample batch.
Pharmacokinetic parameters.
Cmax is the observed maximum serum concentration for each individual, and Tmax is the time at which Cmax is recorded. The elimination rate constant (kel) was determined from the concentration versus time curve. The area under the time-concentration curve (AUC) from 0 to 6 hours (AUC0–6) was calculated according to the linear trapezoidal rule. AUC0-∞ and clearance were also calculated.
Statistical methods.
The data were summarized as means and standard deviations (SD). The Z-score for weight for age was calculated according to WHO growth charts (27).
For binominal data, differences between groups were determined using Fisher's exact test. The Spearman rank correlation coefficient described associations between continuous variables. A mixed-model repeated-measures analysis of variance (ANOVA) was performed to evaluate the influence of HIV infection and to assess the differences for the pharmacokinetic parameters between the two treatment groups and following 1 and 4 months of therapy.
RESULTS
A total of 31 children were enrolled; 15 children received a regimen that included ETH and RMP, and 16 received an ETH-containing regimen without RMP. For one 2-year-old child, RMP was added only after inclusion in the study and after completion of the first study day. For one child, the 4-month data point was missing because of failed venipuncture. All HIV-infected children were established on antiretroviral therapy (at least 2 to 4 weeks of therapy) at enrollment.
The demographics, diagnostic criteria, and clinical features of participants are shown in Table 1. A RMP-including treatment regimen is used in the treatment of TBM but usually not in the treatment of MDR-TB, explaining the different clinical features in the two study groups.
Table 1.
Demographic, clinical, and radiological features of children by age group and treatment regimens
| Characteristica | No. of children: |
||
|---|---|---|---|
| <2 yr (n = 10) without/with RMP | 2 to 6 yr (n = 11) without/with RMP | 6 to 12 yr (n = 10) without/with RMP | |
| Total | 5/5 | 6/5 | 5/5 |
| Mean age (SD) | 1.0 (0.7)/1.0 (0.6) | 2.7 (1.2)/3.2 (1.3) | 9.3 (2.1)/8.5 (1.7) |
| Female | 3/3 | 4/1 | 5/1 |
| Culture of M. tuberculosis or AFB positive | 2/4 | 4/2 | 4/2 |
| Household TB contact | 4/4 | 2/3 | 4/1 |
| HIV infected | 2/2 | 2/2 | 3/1 |
| Mantoux test done | 5/4 | 4/3 | 2/2 |
| Clinical features | |||
| Pulmonary TB | 5/2 | 5/0 | 4/0 |
| Tuberculous meningitis | 0/3 | 0/5 | 0/5 |
| Hepatomegaly at inclusion | 3/1 | 2/2 | 2/1 |
| Nutritional statuses | |||
| Mass < 3rd percentile | 1/0 | 0/2 | 2/1 |
| Mean wt, 1 month (SD) | 8.3 (2.6)/8.7 (2.4) | 13.1 (2.0)/12.8 (2.6) | 26.1 (7.5)/23.7 (2.0) |
| Mean wt, 4 months (SD) | 9.6 (2.6)/9.2 (2.7) | 13.5 (2.1)/13.0 (2.7) | 25.7 (7.3)/23.1 (2.0) |
| Mean MUAC, 1 month (SD) | 13.6 (2.1)/14.1 (2.0) | 16.0 (1.2)/15.6 (0.9) | 17.9 (1.9)/17.6 (0.9) |
| Mean MUAC, 4 months (SD) | 14.4 (2.1)/15.7 (2.2) | 15.8 (1.1)/15.2 (1.2) | 18.0 (2.0)/17.2 (0.6) |
| Mean BMI, 1 month (SD) | 15.8 (3.0)/16.9 (1.7) | 15.8 (1.1)/16.6 (2.0) | 15.5 (2.1)/15.2 (1.2) |
| Mean BMI, 4 months (SD) | 16.9 (2.7)/16.4 (2.6) | 15.7 (0.9)/15.8 (1.6) | 15.2 (2.0)/14.4 (0.6) |
| Radiological featuresb | |||
| Hilar adenopathy | 3/3 | 3/1 | 2/1 |
| Airway compression | 2/0 | 1/1 | 0/1 |
| Alveolar opacification | 4/1 | 4/2 | 2/2 |
| Cavitation | 1/0 | 0/1 | 2/0 |
| Bronchopneumonic opacification | 1/0 | 0/0 | 1/0 |
| Pleural effusion | 1/0 | 1/0 | 0/0 |
SD = standard deviation; AFB = acid-fast bacilli; MUAC = mid-upper-arm circumference; BMI = body mass index (kg/m2).
For one child with tuberculous meningitis, no chest X-ray was available.
ETH pharmacokinetic parameters are displayed in Table 2. The variability of pharmacokinetic parameters at both time points was high (Table 2). Children were on daily treatment with ETH for a mean of 36 days (SD, 7.13 days) at the 1-month sampling point and 120 days (SD, 5.5 days) at the 4-month sampling point, respectively. The mean ETH doses administered at the respective sampling points were 17.8 mg/kg (SD, 2.4 mg/kg) and 17.6 mg/kg (SD, 1.9 mg/kg), respectively.
Table 2.
Pharmacokinetic parameters of ethionamide in children in different age groups on multidrug regimens with and without rifampin
| Pharmacokinetic parameter | Value for childrena: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <2 yr |
2 to 6 yr |
6 to 12 yr |
||||||||||
| No. of children on ETH | ETH | No. of children on ETH+RMP | ETH+RMP | No. of children on ETH | ETH | No. of children on ETH+RMP | ETH+RMP | No. of children on ETH | ETH | No. of children on ETH+RMP | ETH+RMP | |
| 1 month of therapy | ||||||||||||
| Cmax (μg/ml) | 5 | 3.79 (1.59) | 5 | 3.91 (1.61) | 6 | 4.43 (1.23) | 5 | 3.58 (1.36) | 5 | 3.62 (1.30) | 5 | 5.44 (1.24) |
| Tmax (h) | 5 | 0.97 (0.9–1.0) | 5 | 0.98 (0.9–1.3) | 6 | 1.11 (0.9–2.1) | 5 | 1.00 (1.0–1.1) | 5 | 2.00 (1.0–3.0) | 5 | 1.97 (1.0–2.1) |
| kel (1/h) | 5 | 0.94 (0.58) | 5 | 0.68 (0.23) | 6 | 0.57 (0.15) | 5 | 0.71 (0.27) | 5 | 0.51 (0.67) | 5 | 0.53 (0.09) |
| t1/2 (h) | 5 | 0.92 (0.40) | 5 | 1.11 (0.33) | 6 | 1.30 (0.46) | 5 | 1.08 (0.37) | 5 | 1.38 (0.20) | 5 | 1.32 (0.19) |
| AUC0–6 (mg · h/liter) | 5 | 7.60 (3.50) | 5 | 8.41 (3.71) | 6 | 10.61 (5.59) | 5 | 8.31 (3.51) | 5 | 12.33 (4.34) | 5 | 13.94 (4.87) |
| AUC0–∞ (mg · h/liter) | 5 | 7.84 (3.74) | 5 | 8.75 (3.87) | 6 | 11.51 (6.90) | 5 | 8.72 (3.90) | 5 | 13.54 (4.96) | 5 | 15.04 (5.50) |
| Clearance (no. of liters/h) | 5 | 22.01 (9.89) | 5 | 19.5 (8.59) | 6 | 24.29 (8.93) | 5 | 31.89 (14.80) | 5 | 34.49 (9.38) | 5 | 30.61 (11.01) |
| 4 months of therapy | ||||||||||||
| Cmax (μg/ml) | 5 | 2.78 (1.15) | 4 | 2.15 (1.19) | 6 | 4.67 (1.02) | 6 | 4.28 (1.98) | 5 | 5.06 (2.83) | 5 | 6.37 (2.37) |
| Tmax (h) | 5 | 0.97 (0.9–1.0) | 4 | 0.99 (1.0–2.0) | 6 | 1.90 (1.0–3.2) | 6 | 1.96 (1.0–3.0) | 5 | 1.97 (1.0–3.0) | 5 | 1.98 (1.0–3.0) |
| kel (1/h) | 5 | 0.58 (0.12) | 4 | 1.34 (1.41) | 6 | 0.46 (0.15) | 6 | 0.63 (0.25) | 5 | 0.43 (0.09) | 5 | 0.43 (0.14) |
| t1/2 (h) | 5 | 1.22 (0.24) | 4 | 0.87 (0.45) | 6 | 1.63 (0.56) | 6 | 1.30 (0.61) | 5 | 1.68 (0.33) | 5 | 1.76 (0.52) |
| AUC0–6 (mg · h/liter) | 5 | 6.38 (2.38) | 4 | 4.82 (2.71) | 6 | 12.84 (1.52) | 6 | 13.14 (7.69) | 5 | 15.25 (8.22) | 5 | 20.43 (9.74) |
| AUC0–∞ (mg · h/liter) | 5 | 6.67 (2.33) | 4 | 4.97 (2.82) | 6 | 14.51 (2.82) | 6 | 14.74 (8.88) | 5 | 17.30 (8.67) | 5 | 24.92 (13.64) |
| Clearance (no. of liters/h) | 5 | 25.68 (8.56) | 4 | 37.98 (19.25) | 6 | 16.97 (3.29) | 6 | 25.70 (21.70) | 5 | 30.90 (14.41) | 5 | 21.71 (12.76) |
Shown as mean values (SD), except for Imax, shown as median values (range).
Low maximum serum levels (Cmax < 2.5 μg/ml) were found in 4 children (2 were <2 years of age) at the 1-month sampling and in 7 children (5 were <2 years of age) following 4 months of therapy. Only one child had a Cmax below 2.5 μg/ml at both time points. Cmax ranged between 2.5 μg/ml and 5.0 μg/ml in 15 children (48%) at 1 month and in 13 children (42%) following 4 months of therapy.
HIV coinfection was associated with reduced exposure to ETH at both time points (1 month/4 months, Cmax P = 0.002/0.023, AUC0–6 P = 0.523/0.047; ANOVA); there was no HIV-associated delay in absorption or elimination (1 month/4 months, Tmax P = 0.594/0.970, half-life [t1/2] P = 0.435/0.602).
We found no association between body mass index, mid-upper-arm circumference, or weight-for-age Z scores and any ETH pharmacokinetic parameters (data not shown).
Differences between age groups.
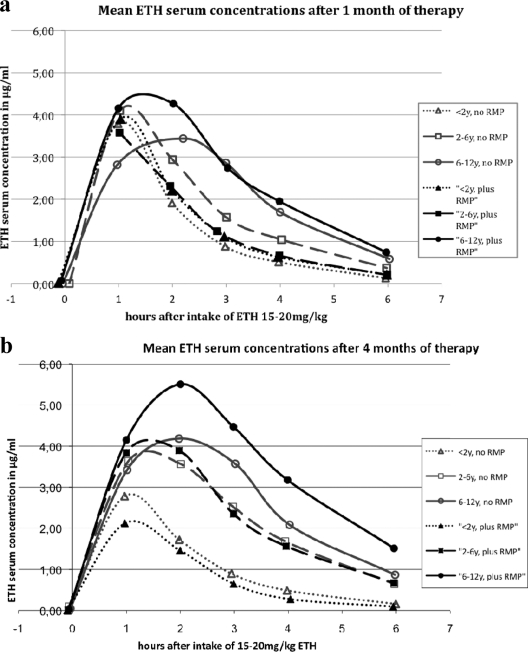
Mean ETH serum concentrations are shown by treatment regimens depicted in Fig. 1a and b, and the corresponding pharmacokinetic parameters appear in Table 2.
Fig. 1.
(a and b) Mean ETH serum concentrations after oral intake of 15 to 20 mg/kg ETH in children of different age groups with or without concomitant treatment with rifampin (RMP). y, years.
Children in both treatment groups (n = 31) were therefore combined for analysis of pharmacokinetic parameters in different age groups.
After month 1 [referred to by subscripted “(1)”], younger children were exposed to lower ETH concentrations than older children. AUC0–6(1) in children of <2 years of age was lower than that in children 6 to <12 years (P = 0.035 and 0.013, respectively) (Table 2). While the differences in Cmax(1) between children of <2 years of age and older children were slight (P = 0.756 and 0.2922, respectively), absorption [Tmax(1), P = 0.372 and 0.004, respectively] and elimination [t1/2(1), P = 0.215 and 0.038, respectively; kel(1), P = 0.197 and 0.036, respectively] occurred more rapidly in younger children.
At month 4 [referred to by subscripted “(4)”], differences between the age groups (children of <2 years of age and older children) became more pronounced [AUC0–6(4), P = 0.027 and 0.001, respectively]. Cmax(4) in children younger than 2 years of age was significantly lower than that in older children (P = 0.034 and 0.002, respectively). Again, absorption [Tmax(4), P = 0.033 and 0.08, respectively] and elimination [t1/2(4), P = 0.168 and 0.051, respectively; kel(4), P = 0.140 and 0.002, respectively] occurred more rapidly in younger children than in older children.
Age correlated significantly with Tmax(1) (r = 0.43, P = 0.02), AUC0–6(1) (r = 0.50, P = 0.001), and clearance(1) (r = 0.44, P = 0.01) at month 1, while the correlations with Cmax(1) (r = 0.18, P = 0.32), kel(1) (r = −0.34, P = 0.06), and t1/2(1) (r = 0.34, P = 0.06) were not statistically significant. At month 4, age correlated significantly with Tmax(4) (r = 0.47, P = 0.01), AUC0–6(4) (r = 0.63, P = 0.001), Cmax(4) (r = 0.60, P = 0.00), kel(4) (r = 0.43, P = 0.02), and t1/2(4) (r = 0.43, P = 0.02) but was not correlated with ETH clearance (2) (r = −0.10, P = 0.60).
Differences: RMP/no RMP.
No differences in ETH pharmacokinetic parameters were detected at either sampling point between children receiving a RMP-including regimen or not (1 month/4 months of therapy), as follows: Cmax, P = 0.530/0.859; Tmax, P = 0.304/0.748; AUC0–6, P = 0.835/0.627; kel, P = 0.735/0.109; t1/2, P = 0.891/0.102; and clearance, P = 0.833/0.372.
Similar results were obtained in the RMP/no RMP analysis stratified by age (data not shown).
Differences in ETH levels over time.
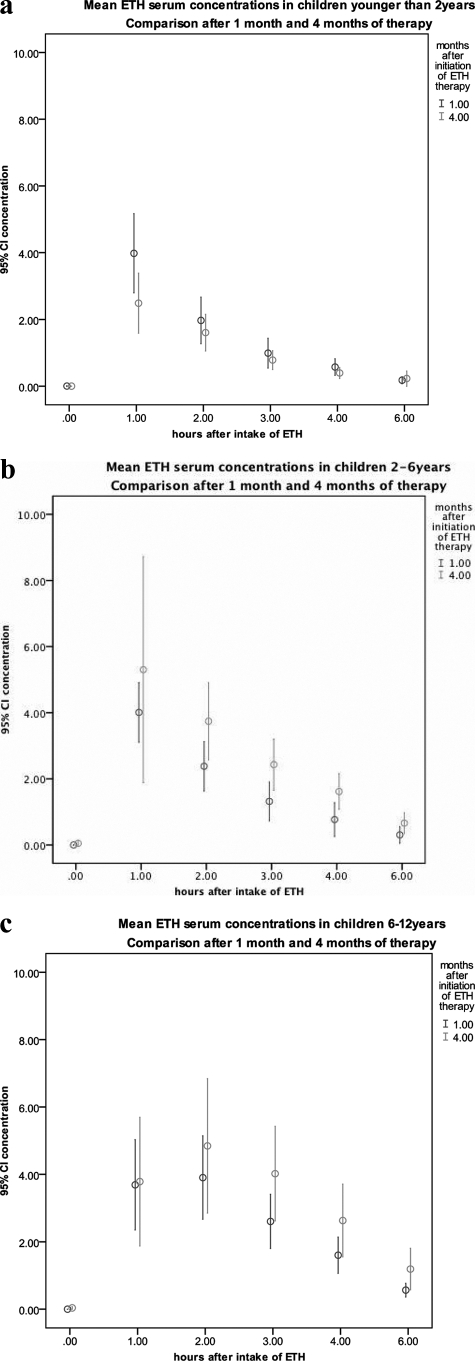
Differences in ETH serum levels at 1 and 4 months of therapy are shown in Fig. 2a to c by age group.
Fig. 2.
(a to c) Mean ETH serum levels before and after intake of ETH after 1 and 4 months of antituberculosis therapy including ETH. Both children with and without RMP are combined in graphs (n = 31). CI, confidence interval.
Taking all children together, there was no difference between the two time points for Cmax (P = 0.6934), AUC0–6 (P = 0.131), kel (P = 0.887), and clearance (P = 0.949). Tmax was significantly lower at 1 month [mean Tmax(1) versus mean Tmax(4), 78 min versus 98 min, respectively; P = 0.048], as was t1/2 [t1/2(1) versus t1/2(4), 71 min versus 83 min, respectively; P = 0.035].
DISCUSSION
With an ETH dose as currently recommended by WHO, the majority of children achieved sufficient serum levels. Children younger than 2 years of age were exposed to lower ETH concentrations than older children receiving the same mg/kg dose. This may imply a need for a higher dosage in this age group. HIV coinfection led to lower ETH serum levels.
Neither comedication with RMP nor the duration of ETH treatment influenced ETH pharmacokinetic parameters in children.
Our study documents high variability in ETH serum levels in children receiving a standard oral dose of ETH of 15 to 20 mg/kg body weight. In adults, significant intra- and interindividual variations in ETH serum concentrations have been reported (2, 19, 21), consistent with our findings. Although in the youngest children, the observed variability might be partly attributable to difficulties in taking medication, we observed similar variability in older children, where no such difficulties were experienced.
ETH peak serum concentrations and the resulting drug exposures were lower in younger children. In up to 50% of children (5 of 10) younger than 2 years of age, recommended serum levels of ETH were not achieved. Lower levels of first-line antituberculosis drugs have previously been reported in children than in adults; these have been attributed to changes in the relative size of body compartments and body surface area (BSA) and changes in the ability to absorb, metabolize, and excrete drugs (1, 4, 7, 25, 26, 29). While the time until maximum serum concentrations were reached was similar (Tmax, 1 to 2 h), Cmax was considerably lower in children younger than 2 years than in older children or adults. After the first weeks of life, gastrointestinal absorption is less relevant for the bioavailability of a drug than the volume of distribution and first-pass metabolism (3). ETH is widely distributed into body tissues and fluids, resulting in a high volume of distribution in adults and children (28). After maturation of liver enzymes, the rate of metabolism is determined mainly by liver growth, which correlates well with BSA. Most hepatic enzymes are mature by the age of 1 year (3). Therefore, a comparatively larger liver size and correspondingly higher hepatic blood flow in young children may account for the high first-pass effect, resulting in reduced bioavailability of ETH. The same principles are the reason for the more rapid elimination of ETH in children than in adults. In studies of ETH pharmacokinetics in adults, a kel of 0.4/h and a corresponding elimination half-life of 1.6 to 1.8 h has been reported (2, 29). In our study, the mean half-life in children below 2 years of age was approximately 1 h and increased with age. Because ETH metabolism is predominantly by hepatic sulfoxidation, ETH dosing according to BSA should therefore be considered to allow for age-related changes in liver size.
Comedication with RMP did not influence the pharmacokinetics of ETH in the present study. RMP is a strong inducer of several enzymes, including different monooxygenases (17). Reduced bioavailability of several drugs (e.g., antiretroviral drugs, oral contraceptives, and oral anticoagulants) when given in combination with RMP has been reported (5, 16), and reduced serum levels of antituberculosis drugs in the context of an RMP-containing treatment regimen has therefore been a concern. RMP does not influence the bioavailability of isoniazid (18). The monooxygenases responsible for ETH sulfoxidation are not part of the P450 system. We did not find any evidence that these monooxygenases are induced by RMP, which is, to our knowledge, the first study to have assessed this potential drug interaction.
Accumulation or self-induction of metabolism during continuous antituberculosis therapy might alter the pharmacokinetic parameters of a drug over time. For ETH, both the absorption and elimination seemed to be delayed after 4 months of therapy. The reason for this is unclear. Slowing down of the enzyme activity responsible for ETH metabolism over time might be a possible reason but has not yet been described. However, since children were exposed to the same amount of drug, measured by the AUC at the beginning of and during continuous therapy, the clinical relevance of this finding is probably minor.
Although not part of our primary study aims, we found that HIV-infected children were exposed to lower ETH concentrations than their HIV-uninfected counterparts. Reduced serum levels of several antituberculosis drugs in HIV-infected adults and children have been reported and have been attributed to malabsorption caused by drug-drug interactions, diarrhea, and/or concurrent gastrointestinal infections (6, 11, 13, 23).
Despite the observed differences, the mean ETH serum concentrations in children of all age groups were above the recommended serum level of 2.5 μg/ml following a standard oral dose of ETH of 15 to 20 mg/kg. In older children and adults, ETH causes dose-dependent serious gastrointestinal irritability and clinically, dosing is therefore driven by tolerance rather than efficacy. Lower mg/kg doses in older children might therefore increase patients' tolerability and compliance to ETH-inclusive treatment regimes without reducing the potential therapeutic efficacy. Despite adequate doses in most children, some possible concerns regarding lower ETH doses remain, as follows: a considerable proportion of children in our study were exposed to low ETH serum concentrations, and after 4 months of therapy, 7 of 31 children (5 aged <2 years) did not achieve the recommended ETH serum levels. A previous study of children with TBM compared ETH concentrations in the cerebrospinal fluid (CSF) after an oral dose of ETH at 15 mg/kg versus 20 mg/kg (8). After the 15-mg/kg dose, the recommended ETH level was achieved in the CSF in very few children (27%); therefore, a single daily dose of 20 mg/kg ETH was recommended for treatment of TBM (8). There is evidence that ETH can produce a bactericidal effect in concentrations of 2.5 to 5.0 μg/ml in vitro, with a 99% killing of M. tuberculosis (15). In vivo, ETH is metabolized mainly to ETH-sulfoxide, which is less stable than ETH but also has antimycobacterial activity and might add to the therapeutic efficacy of ETH (12). At this stage, it is unknown whether higher in vivo ETH serum concentrations (e.g., above 2.5 μg/ml) increase the bactericidal activity or if lower ETH levels are sufficient in the presence of this active metabolite. A limitation of our study is that adverse effects were not studied systematically. Our findings in this relatively small study group also need to be validated in further studies.
In conclusion, ETH at the recommended oral dose of 15 to 20 mg/kg leads to mean serum levels at or above the recommended concentration in the majority of children of all ages. ETH levels are not influenced by concomitant treatment with RMP and do not change substantially during treatment. Nevertheless, the variability in ETH serum levels is high, and inadequate levels in some children were achieved. Younger children and HIV-infected children are at higher risk for subtherapeutic serum levels, and higher dosages can be considered if clinically tolerated. The recommended dose of 15 to 20 mg/kg seems appropriate in older children.
Footnotes
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Anderson G. D., Lynn A. M. 2009. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy 29:680–690 [DOI] [PubMed] [Google Scholar]
- 2. Auclair B., Nix D. E., Adam R. D., James G. T., Peloquin C. A. 2001. Pharmacokinetics of ethionamide administered under fasting conditions or with orange juice, food, or antacids. Antimicrob. Agents Chemother. 45:810–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartelink I. H., Rademaker C. M., Schobben A. F., van den Anker J. N. 2006. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin. Pharmacokinet. 45:1077–1097 [DOI] [PubMed] [Google Scholar]
- 4. Blake M. J., et al. 2006. Pharmacokinetics of rifapentine in children. Pediatr. Infect. Dis. J. 25:405–409 [DOI] [PubMed] [Google Scholar]
- 5. Bolt H. M. 2004. Rifampicin, a keystone inducer of drug metabolism: from Herbert Remmer's pioneering ideas to modern concepts. Drug Metab. Rev. 36:497–509 [DOI] [PubMed] [Google Scholar]
- 6. Chideya S., et al. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 48:1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donald P. R., Maher D., Maritz J. S., Qazi S. 2006. Ethambutol dosage for the treatment of children: literature review and recommendations. Int. J. Tuberc. Lung Dis. 10:1318–1330 [PubMed] [Google Scholar]
- 8. Donald P. R., Seifart H. I. 1989. Cerebrospinal fluid concentrations of ethionamide in children with tuberculous meningitis. J. Pediatr. 115:483–486 [DOI] [PubMed] [Google Scholar]
- 9. Dover L. G., et al. 2004. Crystal structure of the TetR/CamR family repressor Mycobacterium tuberculosis EthR implicated in ethionamide resistance. J. Mol. Biol. 340:1095–1105 [DOI] [PubMed] [Google Scholar]
- 10. Eule H. 1965. Ethionamide concentration in the blood and urine of healthy persons and lung patients. Beitr. Klin. Erforsch. Tuberk. Lungenkr. 132:339–342 (In German.) [PubMed] [Google Scholar]
- 11. Graham S. M., et al. 2006. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob. Agents Chemother. 50:407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grunert M., Werner E., Iwainsky H., Eule H. 1968. Changes in the ethioniamide and its sulfoxides in vitro. Beitr. Klin. Erforsch. Tuberk. Lungenkr. 138:68–82 (In German.) [PubMed] [Google Scholar]
- 13. Gurumurthy P., et al. 2004. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin. Infect. Dis. 38:280–283 [DOI] [PubMed] [Google Scholar]
- 14. Heifets L. 1988. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am. Rev. Respir. Dis. 137:1217–1222 [DOI] [PubMed] [Google Scholar]
- 15. Heifets L. B., Lindholm-Levy P. J., Flory M. 1991. Comparison of bacteriostatic and bactericidal activity of isoniazid and ethionamide against Mycobacterium avium and Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 143:268–270 [DOI] [PubMed] [Google Scholar]
- 16. Heimark L. D., Gibaldi M., Trager W. F., O'Reilly R. A., Goulart D. A. 1987. The mechanism of the warfarin-rifampin drug interaction in humans. Clin. Pharmacol. Ther. 42:388–394 [DOI] [PubMed] [Google Scholar]
- 17. Henderson M. C., Siddens L. K., Morre J. T., Krueger S. K., Williams D. E. 2008. Metabolism of the anti-tuberculosis drug ethionamide by mouse and human FMO1, FMO2 and FMO3 and mouse and human lung microsomes. Toxicol. Appl. Pharmacol. 233:420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holdiness M. R. 1984. Clinical pharmacokinetics of the antituberculosis drugs. Clin. Pharmacokinet. 9:511–544 [DOI] [PubMed] [Google Scholar]
- 19. Hughes I. E., Smith H. 1962. Ethionamide: its passage into the cerebrospinal fluid in man. Lancet i:616–617 [DOI] [PubMed] [Google Scholar]
- 20. Maglich J. M., et al. 2002. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 62:638–646 [DOI] [PubMed] [Google Scholar]
- 21. Mattila M. J., Koskinen R., Takki S. 1968. Absorption of ethionamide and prothionamide in vitro and in vivo. Ann. Med. Intern. Fenn. 57:75–79 [PubMed] [Google Scholar]
- 22. Peloquin C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183 [DOI] [PubMed] [Google Scholar]
- 23. Peloquin C. A., et al. 1996. Low antituberculosis drug concentrations in patients with AIDS. Ann. Pharmacother. 30:919–925 [DOI] [PubMed] [Google Scholar]
- 24. Schaaf H. S., Marais B. J., Hesseling A. C., Brittle W., Donald P. R. 2009. Surveillance of antituberculosis drug resistance among children from the Western Cape Province of South Africa—an upward trend. Am. J. Public Health 99:1486–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schaaf H. S., et al. 2005. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch. Dis. Child. 90:614–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thee S., Detjen A., Quarcoo D., Wahn U., Magdorf K. 2007. Ethambutol in paediatric tuberculosis: aspects of ethambutol serum concentration, efficacy and toxicity in children. Int. J. Tuberc. Lung Dis. 11:965–971 [PubMed] [Google Scholar]
- 27. WHO 2011, posting date Child growth standards. WHO, Geneva, Switzerland: http://www.who.int/childgrowth/standards/weight_for_age/en/index.html [Google Scholar]
- 28. WHO 2009, posting date Dosing instructions for the use of currently available fixed-dose combination TB medicines for children. WHO, Geneva, Switzerland: www.who.int/entity/tb/challenges/interim_paediatric_fdc_dosing_instructions_sept09.pdf [Google Scholar]
- 29. Zhu M., et al. 2002. Population pharmacokinetics of ethionamide in patients with tuberculosis. Tuberculosis (Edinb.) 82:91–96 [DOI] [PubMed] [Google Scholar]