Abstract
The efficacies of many antimicrobial peptides are greatly reduced under high salt concentrations, limiting their development as pharmaceutical compounds. Here, we describe an easy strategy to increase salt resistance of antimicrobial peptides by replacing tryptophan or histidine residues with the bulky amino acids β-naphthylalanine and β-(4,4′-biphenyl)alanine. The activities of the salt-sensitive peptide P-113 were diminished at high salt concentrations, whereas the activities of its β-naphthylalanine and β-(4,4′-biphenyl)alanine-substituted variant were less affected.
TEXT
Antimicrobial peptides play important roles in the host innate defense mechanism by interacting and permeabilizing microbial membranes (21). With an increase of antibiotic resistance, the potential for the development of antimicrobial peptides as novel therapeutic agents could overcome the problem of resistance (5).
The development of antimicrobial peptides has been hindered by several problems. One of these problems is salt sensitivity (13). The efficacy of human β-defensin-1 is greatly reduced in the presence of high salt concentrations in bronchopulmonary fluids in cystic fibrosis patients (4). Similar problems were observed in the clinically active histidine-rich peptide P-113, indolicidins, gramicidins, bactenecins, and magainins (12, 14, 17, 20). A number of studies have been reported on the design of salt-resistant antimicrobial peptides. However, most of them were focused on overall structure modifications, such as structure rigidity, helix stability, hydrophobicity, and amphipathicity (2, 3, 6, 12, 13, 15, 16).
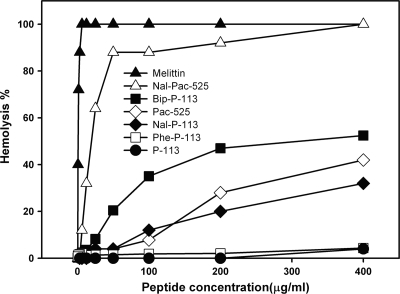
Previously, we found that a novel Trp-rich peptide, Ac-KWRRWVRWI-NH2, designated Pac-525, demonstrated improved activity against both bacteria and fungi and showed reduced hemolytic activity (18). Nal-Pac-525, with all of its tryptophans replaced by the bulky amino acid β-naphthylalanine (7), was shown to have a higher antimicrobial activity than Pac-525 (19). More importantly, unlike Pac-525, the antifungal activity of Nal-Pac-525 against fluconazole-resistant fungal pathogens was not blocked by high-salt incubation conditions (17). However, Nal-Pac-525 has a high hemolytic activity that prohibits its further clinical application (Fig. 1). Thus, the advantages and disadvantages of bulky amino acid substitution to salt-resistant antimicrobial peptides remain to be elucidated. P-113 is a 12-amino-acid histidine-rich peptide derived from the saliva protein histatin 5. The anti-Candida activity of P-113 has been documented previously (14). Recently, a clinical trial involving HIV patients showed that P-113 has a good outcome for oral candidiasis therapy, but the activity of P-113 is restricted to a low-salt condition, limiting its application (8).
Fig. 1.
Hemolytic activities of P-113 (●), Phe-P-113 (□), Nal-P-113 (◆), Bip-P-113 (■), Pac-525 (♢), and Nal-Pac-525 (▵). Melittin (▴) was used as a control.
Based on the studies of Pac-525 and Nal-Pac-525, it is hypothesized that the substitution of tryptophan residues by the bulky amino acid β-naphthylalanine may generate a potent peptide with improved antimicrobial activity and salt resistance. β-Naphthylalanine residues may position themselves deeper into the bacterial and fungal cell membranes, making the peptide more efficient in disrupting the membranes, hence compensating the competition from the cations to the negatively charged microbial cell surface. However, the hemolytic activity of Nal-Pac-525 increases dramatically with the β-naphthylalanine substitutions (Fig. 1). These observations lead to the hypothesis that the replacement of the aromatic residues with bulky aromatic amino acids of salt-sensitive and low-hemolytic antimicrobial peptides may result in salt-resistant peptides with slight increases of their hemolytic activities.
The histatin derivative P-113, AKRHHGYKRKFH-NH2, was used to test this hypothesis. We have designed and synthesized Nal-P-113, with His 4, 5, and 12 replaced by β-naphthylalanines (19). Bacterial and fungal strains from ATCC and clinical isolates (17) used in this study are listed in Tables 1, 2, and 3. The antibacterial activities of P-113 and Nal-P-113 were determined by the standard broth microdilution method of the National Committee for Clinical Laboratory Standards with Mueller-Hinton (MH) broth and LYM broth (14). The anti-Candida activities of fluconazole, P-113, and Nal-P-113 were determined in LYM broth medium (14) with different salt concentrations. The MIC value is the lowest concentration of peptide at which there was no change in optical density. All tests were measured in triplicate. The hemolytic activities of the peptides were determined from hemolysis against human red blood cells (hRBC) (18). The MIC values of Nal-P-113 were found to be more potent than those of P-113 (Tables 1 and 2), with only a slight increase of hemolysis of hRBCs (Fig. 1).
Table 1.
MIC values for ampicillin, P-113, Phe-P-113, Nal-P-113, and Bip-P-113 in different concentrations of NaCl
| Species | MIC (μg/ml)a |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MH broth |
LYM broth |
|||||||||||||||||||||||||
| Control |
NaCl concn (mM) |
|||||||||||||||||||||||||
| 50 |
100 |
200 |
300 |
|||||||||||||||||||||||
| AP | P-113 | Phe | Nal | Bip | AP | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | |
| Escherichia coli ATCC 25922 | 25 | >50 | >50 | 12.5 | 12.5 | 25 | 12.5 | 12.5 | 3.1 | 6.25 | 50 | 50 | 3.1 | 12.5 | 50 | >50 | 12.5 | 12.5 | >50 | >50 | 25 | 12.5 | >50 | >50 | 50 | 12.5 |
| Staphylococcus aureus ATCC 25923 | 3.1 | >50 | >50 | 6.25 | 3.1 | 12.5 | 12.5 | 6.25 | 1.56 | 3.1 | 50 | 12.5 | 1.56 | 6.25 | 50 | 50 | 3.1 | 12.5 | >50 | >50 | 12.5 | 12.5 | >50 | >50 | 50 | 25 |
| S. aureus ATCC 29213 | 12.5 | >50 | >50 | 6.25 | 6.25 | 12.5 | 12.5 | 6.25 | 1.56 | 3.1 | 50 | 25 | 1.56 | 6.25 | 50 | >50 | 3.1 | 6.25 | >50 | >50 | 6.25 | 6.25 | >50 | >50 | 25 | 12.5 |
| S. aureus ATCC 19636 | 25 | >50 | >50 | 6.25 | 12.5 | 6.25 | 50 | 25 | 6.25 | 6.25 | 50 | 50 | 12.5 | 6.25 | 50 | >50 | 25 | 6.25 | >50 | >50 | 50 | 12.5 | >50 | >50 | 50 | 12.5 |
| Pseudomonas aeruginosa ATCC 27853 | X | >50 | >50 | 50 | 50 | X | 25 | 25 | 1.56 | 3.1 | 50 | 50 | 1.56 | 3.1 | 50 | 50 | 12.5 | 6.25 | >50 | >50 | 50 | 6.25 | >50 | >50 | >50 | 12.5 |
| P. aeruginosa ATCC 9027 | X | >50 | >50 | 50 | 50 | X | 12.5 | 25 | 3.1 | 3.1 | 50 | >50 | 6.25 | 6.25 | 50 | >50 | 50 | 6.25 | >50 | >50 | 50 | 6.25 | >50 | >50 | >50 | 50 |
X, ampicillin resistant; AP, ampicillin; Phe, Phe-P-113; Nal, Nal-P-113; Bip, Bip-P-113.
Table 2.
MIC values for ampicillin, P113, Phe-P-113, Nal-P-113, and Bip-P-113 in different pH conditions and concentrations of MgCl2
| Species | MIC (μg/ml) in LYM brotha |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
MgCl2 concn (mM) |
pH condition |
|||||||||||||||||||||||||||
| 0.5 |
1.5 |
2.5 |
6.5 |
5.5 |
4.5 |
||||||||||||||||||||||||
| AP | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | P-113 | Phe | Nal | Bip | |
| E. coli ATCC 25922 | 25 | 12.5 | 12.5 | 3.1 | 6.25 | 12.5 | 25 | 3.1 | 6.25 | 25 | 50 | 6.25 | 6.25 | 25 | >50 | 12.5 | 6.25 | 12.5 | 50 | 6.25 | 3.1 | 12.5 | >50 | 12.5 | 12.5 | >50 | >50 | 12.5 | 12.5 |
| S. aureus ATCC 25923 | 12.5 | 12.5 | 6.25 | 1.56 | 3.1 | 50 | 6.25 | 3.1 | 3.1 | 50 | >50 | 3.1 | 3.1 | 50 | >50 | 3.1 | 3.1 | 12.5 | 6.25 | 3.1 | 3.1 | 50 | 50 | 12.5 | 3.1 | 6.25 | 50 | 6.25 | 3.1 |
| S. aureus ATCC 29213 | 12.5 | 12.5 | 6.25 | 1.56 | 3.1 | 12.5 | 6.25 | 1.56 | 3.1 | 25 | 12.5 | 1.56 | 3.1 | 25 | >50 | 1.56 | 3.1 | 12.5 | >50 | 1.56 | 3.1 | 6.25 | >50 | 12.5 | 6.25 | 3.1 | 50 | 25 | 6.25 |
| S. aureus ATCC 19636 | 6.25 | 50 | 25 | 6.25 | 6.25 | >50 | 25 | 12.5 | 6.25 | >50 | 25 | 12.5 | 6.25 | >50 | >50 | 25 | 6.25 | >50 | 25 | 3.1 | 6.25 | >50 | >50 | 6.25 | 6.25 | >50 | >50 | 6.25 | 3.1 |
| P. aeruginosa ATCC 27853 | X | 25 | 25 | 1.56 | 3.1 | 50 | 50 | 3.1 | 3.1 | 50 | >50 | 3.1 | 6.25 | 50 | >50 | 6.25 | 12.5 | 12.5 | >50 | 1.56 | 6.25 | 50 | >50 | 3.1 | 6.25 | >50 | >50 | 12.5 | 6.25 |
| P. aeruginosa ATCC 9027 | X | 12.5 | 25 | 3.1 | 3.1 | >50 | 50 | 6.25 | 3.1 | >50 | 50 | 6.25 | 6.25 | >50 | >50 | 12.5 | 6.25 | >50 | >50 | 6.25 | 25 | >50 | >50 | 12.5 | 25 | >50 | >50 | 25 | 6.25 |
X, ampicillin resistant; AP, ampicillin; Phe, Phe-P-113; Nal, Nal-P-113; Bip, Bip-P-113.
Table 3.
Susceptibilities of Candida strains to fluconazole, P-113, and Nal-P-113 in modified LYM medium
| Candida sp. | Source | Strain | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluconazole | Control |
50 mM NaCl |
100 mM NaCl |
150 mM NaCl |
|||||||
| P-113 | Nal | P-113 | Nal | P-113 | Nal | P-113 | Nal | ||||
| C. krusei | ATCC 6258 | YLO6 | 32 | 6.25 | 6.25 | 6.25 | 6.25 | 100 | 6.25 | 100 | 12.5 |
| C. parapsilosis | ATCC 22019 | YLO7 | 8 | 6.25 | 6.25 | 50 | 12.5 | >100 | 12.5 | >100 | 25 |
| C. glabrata | ATCC 9003 | YLO8 | 16 | 12.5 | 6.25 | >100 | 25 | >100 | 25 | >100 | 25 |
| C. albicans | ATCC 90028 | YLO12 | 1 | 6.25 | 6.25 | 25 | 12.5 | 100 | 12.5 | >100 | 12.5 |
| C. tropicalis | ATCC 13803 | YLO86 | >64 | 3.1 | 6.25 | 3.1 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 |
| C. albicans | HIV patient | YH050001 | 2 | 6.25 | 6.25 | 25 | 12.5 | 100 | 12.5 | >100 | 12.5 |
| C. albicans | HIV patient | YH050005 | 1 | 6.25 | 6.25 | 50 | 25 | >100 | 25 | >100 | 25 |
| C. tropicalis | HIV patient | YH050007 | >64 | 3.1 | 6.25 | 3.1 | 6.25 | 25 | 6.25 | 25 | 12.5 |
| C. tropicalis | HIV patient | YH050013 | >64 | 3.1 | 6.25 | 3.1 | 6.25 | 50 | 6.25 | 50 | 12.5 |
| C. albicans | HIV patient | YH050072 | >64 | 6.25 | 6.25 | 25 | 25 | >100 | 25 | >100 | 25 |
| C. krusei | HIV patient | YH050075 | 64 | 6.25 | 6.25 | 25 | 12.5 | >100 | 12.5 | >100 | 12.5 |
| C. dubliniensis | HIV patient | YH050092 | 0.5 | 6.25 | 6.25 | 12.5 | 12.5 | >100 | 12.5 | >100 | 12.5 |
| C. glabrata | HIV patient | YH050105 | 16 | 6.25 | 6.25 | 50 | 12.5 | 100 | 25 | >100 | 50 |
| C. tropicalis | HIV patient | YH050114 | >64 | 3.1 | 6.25 | 3.1 | 6.25 | 100 | 6.25 | 100 | 12.5 |
Several studies reported that the efficacy of P-113 is greatly reduced in the presence of high salt concentrations (9–11). As can be seen in Tables 1 and 2, P-113 demonstrates activities against various bacterial strains in the LYM broth medium. However, the activity of P-113 was reduced by the addition of 50 mM NaCl or 0.5 mM MgCl2 into the LYM medium and was further diminished by the addition of 200 mM NaCl or 1.5 mM MgCl2. On the other hand, the MIC values of Nal-P-113 were found to be more potent than P-113 in both Mueller-Hinton broth and modified LYM broth medium. Nal-P-113 still retained its antibacterial activities with 300 mM NaCl or 2.5 mM MgCl2 added.
The anti-Candida activities of fluconazole, P-113, and Nal-P-113 were determined in LYM culture medium under different salt conditions. There were six resistant strains with high fluconazole MICs (≥32 μg/ml). As expected, both P-113 and Nal-P-113 had similar MICs in the LYM medium, ranging from 3.1 to 12.5 μg/ml (Table 3). The activity of P-113 was inhibited by the addition of 100 mM NaCl in the LYM medium and was strongly blocked by the addition of 150 mM NaCl. Again, Nal-P-113 retained its antifungal activities in the media containing high salt concentrations (Table 3).
To compare if other bulky amino acids can achieve similar results, we have synthesized Phe-P-113 and Bip-P-113 with His 4, 5, and 12 replaced by phenylalanine or the bulky amino acid β-(4,4′-biphenyl)alanine (Bip) (7). As expected, the antimicrobial and hemolytic activities of Phe-P-113 are similar to those of P-113, and its activities are diminished under high-salt conditions (Tables 1 and 2). On the other hand, the MIC values of Bip-P-113 are found to be more potent than those of P-113, and Bip-P-113 still retains its antimicrobial activities under high-salt conditions (Tables 1 and 2). Even though Bip-P-113 has slightly higher hemolytic activity than Nal-P-113 (Fig. 1), it exhibits less than 5% hemolytic activity at its effective MICs.
Certain antimicrobial peptides containing potent and broad-spectrum activities against various microbial pathogens are already in clinical trials (5). However, the antimicrobial activities of some agents were found to diminish under physiological and high-salt conditions (1, 15, 22). In this study, the β-naphthylalanine and β-(4,4′-biphenyl)alanine-substituted peptides, Nal-P-113 and Bip-P-113, have potent activity against microbial pathogens, including methicillin-, ampicillin-, and fluconazole-resistant strains. Moreover, the antimicrobial activity is no longer hindered by high salt concentrations.
Acknowledgments
We thank Chi-Cheng Peng, Hung-Lun Chu, and Cheng-Ying Lee for help in some of the experiments.
This work is supported by grants from National Science Council, Republic of China.
Footnotes
Published ahead of print on 18 July 2011.
REFERENCES
- 1. Deslousches B., et al. 2005. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob. Agents Chemother. 49:3208–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deslousches B., et al. 2005. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 49:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedrich C., Scott M. G., Karunaratne N., Yan H., Hancock R. E. W. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldman M. J., et al. 1997. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553–560 [DOI] [PubMed] [Google Scholar]
- 5. Hancock R. E. W., Sahl H. G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotech. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 6. Harwig S. S. L., et al. 1996. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 240:352–357 [DOI] [PubMed] [Google Scholar]
- 7. Haug B. E., Skar M. L., Svendsen J. S. 2001. Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferricin derivatives. J. Pept. Sci. 7:425–432 [DOI] [PubMed] [Google Scholar]
- 8. Helmerhorst E. J., Oppenheim F. G., Choi L., Cheng J. W., Reiner N. E. 2007. Evaluation of a new host-derived synthetic antifungal peptide (PAC-113) in the treatment of oral candidiasis. Int. Meet. Antimicrob. Chemother. Clin. Pract. Italy, poster 9 [Google Scholar]
- 9. Helmerhorst E. J., Van T. Hof W., Veerman E. C. I., Simmons-Smit I., Nieuw Amerongen A. V. 1997. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 326:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jang W. S., Li X. S., Sun J. N., Edgerton M. 2008. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 52:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koshlukova S. E., Lloyd T. L., Araujo M. W. B., Edgerton M. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 274:18872–18879 [DOI] [PubMed] [Google Scholar]
- 12. Lee I. H., Cho Y., Lehrer R. I. 1997. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 65:2898–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park I. Y., et al. 2004. Helix stability confers salt resistance upon helical antimicrobial peptides. J. Biol. Chem. 279:13896–13901 [DOI] [PubMed] [Google Scholar]
- 14. Rothstein D. M., et al. 2001. Anti-Candida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 45:1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rydlo T., Rotem S., Mor A. 2006. Antibacterial properties of dermaseptin S4 derivatives under extreme incubation conditions. Antimicrob. Agents Chemother. 50:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tam J. P., Lu Y. A., Yang J. L. 2000. Design of salt-insensitive glycine-rich antimicrobial peptides with cyclic tricystine structures. Biochemistry 39:7159–7169 [DOI] [PubMed] [Google Scholar]
- 17. Wang C. W., et al. 2009. Increased potency of a novel d-beta-naphthylalanine-substituted antimicrobial peptide against fluconazole-resistant fungal pathogens. FEMS Yeast Res. 9:967–970 [DOI] [PubMed] [Google Scholar]
- 18. Wei S. Y., et al. 2006. Solution structure of a novel tryptophan-rich peptide with bidirectional antimicrobial activity. J. Bacteriol. 188:328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu J. M., et al. 2007. Solution structure of a novel D-naphthylalanine substituted peptide with potential antibacterial and antifungal activities. Biopolymers 88:738–745 [DOI] [PubMed] [Google Scholar]
- 20. Wu M., Maier E., Benz R., Hancock R. E. W. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235–7242 [DOI] [PubMed] [Google Scholar]
- 21. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]
- 22. Zhang L., et al. 2005. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 49:2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]