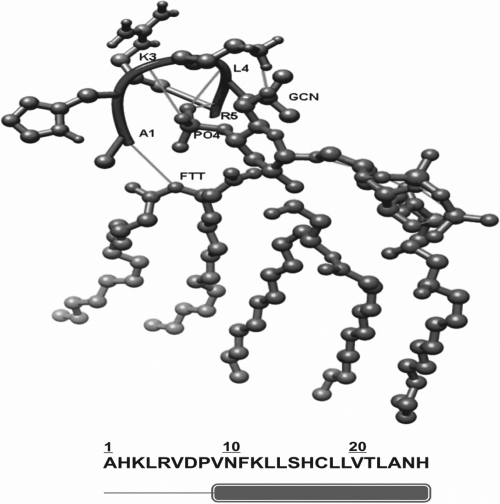
Fig. 1.
Docking of RVFHbαP with LPS. Residues 1 to 8 (Ala-Pro) are in a loop region and residues 9 to 25 (Val-His) are in the helix. Ala1 showed interaction with FTT (3-hydroxy-tetradacanoic acid), Leu4 and Arg5 with PO4, and Lys3 with GCN (3-deoxy-d-glucosamine). The N-terminal region is found to be hydrophilic utill the 9th residue. This region showed interactions with PO4, GCN, and FTT, which were observed mainly between the basic residues and the phosphate ion.