Abstract
Enterotoxigenic Escherichia coli (ETEC) strain H10407 (serotype O78:H11 producing heat-labile toxin [LT], heat-stable toxin [ST], and colonization factor I [CFA/I]) induces reliably high diarrheal attack rates (ARs) in a human challenge model at doses of ≥109 CFU. A descending-dose challenge study was conducted with changes to the standard fasting time and buffer formulation, seeking conditions that permit lower inocula while maintaining reproducibly high ARs. In cohort 1, 20 subjects were fasted overnight and randomized 1:1:1:1 to receive H10407 at doses of 108 CFU with bicarbonate, 108 CFU with CeraVacx, 107 CFU with bicarbonate, or 107 CFU with CeraVacx. Subsequent cohorts received H10407 (107 CFU with bicarbonate) with similar fasting conditions. Cohort 2 included 15 ETEC-naïve volunteers. Cohort 3 included 10 ETEC-naïve volunteers and 10 rechallenged volunteers. In all, 25/35 (71%) ETEC-naïve recipients of 107 CFU of H10407 developed moderate or severe diarrhea (average maximum stool output/24 h = 1,042 g), and most (97%) shed H10407 (maximum geometric mean titer = 7.5 × 107 CFU/gram of stool). Only one of 10 rechallenged volunteers developed diarrhea. These rechallenged subjects had reduced intestinal colonization, reflected by quantitative microbiology of fecal samples. Among the 35 ETEC-naïve subjects, anti-lipopolysaccharide (LPS) O78 serum antibody responses were striking, with positive IgA and IgG antibody responses in 33/35 (94%) and 25/35 (71%), respectively. Anti-heat-labile enterotoxin (LTB) serum IgA and IgG responses developed in 19/35 (54%) and 14/35 (40%) subjects, respectively. Anti-CFA/I serum IgA and IgG responses were detected in 15/35 (43%) and 8/35 (23%) subjects. After the second challenge, participants exhibited blunted anti-LPS and -LTB responses but a booster response to CFA/I. This ETEC model should prove useful in the future evaluation of ETEC vaccine candidates.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is a leading bacterial cause of infectious diarrhea in infants and adults living in developing countries and accounts for approximately 30% of traveler's diarrhea in visitors to these regions (6, 21, 23–26, 28, 29, 35). ETEC strains vary in their pathogenicity as a result of differences in the expression of heat-labile toxin (LT), heat-stable toxin (ST), and several colonization factors (CFAs) that are associated with attachment and colonization in the gut (23, 31). For more than 40 years, human challenge models have been the mainstay for the clinical evaluation of ETEC pathogenesis and immunology (7, 15) and for the assessment of the therapeutic and protective efficacy of antibiotics (2), probiotics (4), and candidate vaccines (18, 19). The recent availability of new resources for ETEC vaccine development has renewed interest in ETEC challenge models. A model with a reliably high attack rate (AR) could provide a vehicle for the evaluation and screening of vaccine efficacy before expensive, long-term field trials are conducted in areas where ETEC is endemic or among at-risk travelers.
Mixed results from prior ETEC vaccine challenge studies have fueled concerns that ETEC challenge models used in the past required higher inocula than those typically encountered in natural exposure, overwhelming vaccine-induced protective immunity (34). Several well-characterized ETEC strains have been used in challenge studies. ETEC strain H10407 has been used most frequently, having been fed to more than 250 subjects, most often for preventive ETEC vaccine proof-of-concept studies (2, 11, 12, 14, 18). ETEC H10407 was originally isolated from an adult with severe diarrhea in Dhaka, Bangladesh. It produces both LT and ST and expresses colonization factor I (CFA/I) (9). ETEC H10407 induces acute, watery diarrhea in otherwise healthy, ETEC-naïve subjects when they are challenged with ≥108 CFU of bacteria (18, 20). The majority of ETEC H10407 challenge studies to date have utilized challenge inocula of 109 CFU in order to ensure diarrheal attack rates of >70%, which are needed to achieve statistical significance during small volunteer studies.
Lower ETEC doses have yielded inconsistent attack rates (15, 17), but lower challenge doses have been used effectively for other well-established enteric disease challenge models, such as Shigella (102 CFU) (13, 32), Vibrio cholerae (105 CFU) (4, 27), and more recently Campylobacter jejuni (105 CFU) (33). Therefore, development of a lower-inoculum ETEC H10407 challenge model could be useful when evaluating ETEC vaccines in the future. Typically, ETEC challenge studies have stipulated that on the day of challenge subjects eat breakfast, fast for 90 min, drink 120 ml of a sodium bicarbonate buffer solution, ingest 1 min later the challenge inoculum dissolved in 30 ml of bicarbonate buffer, and finally fast again for 90 min after the challenge.
Here, we report on a series of studies designed to refine the ETEC H10407 challenge model for future use with volunteer studies. We elected to examine whether we could achieve a high attack rate (AR) while using a lower-inoculum dose if we changed the buffer or if we extended the duration of fasting prior to the challenge. We also wanted to determine if exposure to a first challenge would protect volunteers who were rechallenged using this modified model. Finally, we wanted to measure the serum antibody responses following a first and second challenge.
MATERIALS AND METHODS
Regulatory approval.
The protocol was conducted under BB-IND 12,243 at the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health. Approval to conduct the study was provided by the Western Institutional Review Board (Olympia, WA) for the Johns Hopkins Bloomberg School of Public Health and PATH and by the Institutional Biosafety Committee of the Johns Hopkins Institutions.
Study subjects.
Healthy, 18- to 45-year-old, male or female subjects were recruited for the study using print and electronic media advertisement and by word of mouth. The study was explained to subjects in detail, time was provided for informed consent review, the consent form was individually reviewed with each subject by a member of the study team and signed, and witnessed consent was obtained. In addition, all subjects were required to pass a written assessment of understanding about the study that reviewed the study rationale, procedures, and risks. The prechallenge health status of subjects was assessed by written and oral medical history, physical examination, complete blood count, urinalysis, urine toxicology, blood chemistries, and tests for liver and kidney function, HIV-1, hepatitis B, and hepatitis C. Subjects were excluded if they had significant medical problems detected by history, physical examination, or screening laboratory tests or if an HIV-1, hepatitis B, or hepatitis C test was positive.
Challenge strain.
The challenge strain (H10407) is ETEC serotype O78:H11 and produces both LT and ST and also produces CFA/I. It is sensitive to ampicillin, trimethoprim-sulfamethoxazole, and ciprofloxacin, which are used typically to treat ETEC H10407 infections. A cyclic GMP (cGMP)-quality production cell bank (PCB) for ETEC H10407 was prepared by the Walter Reed Army Institute of Research (WRAIR) Pilot Bioproduction Facility (Silver Spring, MD). The manufacturing information and production records for the PCB of these strains were provided to the FDA under BB-IND-7766 (batch production record 285-000, lot no. 0519). The ETEC H10407 challenge strain was stored in 2-ml cryostorage tubes (1 ml per tube) held at −80°C ± 10°C in the bio-production facility at WRAIR. Cryovials containing organisms from this PCB were transferred on dry ice from WRAIR to the CIR Enterics Research Laboratory, Johns Hopkins Bloomberg School of Public Health, and stored at −80 ± 10°C in a locked and temperature-monitored freezer.
Study design.
The study was conducted in three cohorts between 15 February 2009 and 15 August 2009. Each cohort was admitted as a group into the 30-bed CIR Isolation Unit. For each cohort, ETEC H10407 was used as the challenge strain, and all subjects received a challenge with the virulent organism; none of the subjects received a placebo challenge.
For cohort 1, 20 subjects were given the challenge, but the cohort participants were divided randomly into four subgroups of five subjects per group in a double-blinded manner. Subgroup A received a dose of 2 × 108 CFU with bicarbonate buffer; subgroup B received a dose of 2 × 108 CFU with CeraVacx buffer; subgroup C received a dose of 2 × 107 CFU with bicarbonate buffer; subgroup D received a dose of 2 × 107 CFU with CeraVacx buffer. Based on the high attack rates and similar clinical outcomes observed with all subgroups of cohort 1, the study steering committee decided that subsequent cohorts should receive a dose of 2 × 107 CFU with bicarbonate buffer.
For cohort 2, 15 subjects were administered a dose of 2 × 107 CFU with bicarbonate buffer. For cohort 3, 20 subjects were administered a dose of 2 × 107 CFU with bicarbonate. However, 10 of these subjects in cohort 3 were new subjects who had not participated in the study previously. The other 10 were subjects who had experienced ETEC-associated diarrhea 3 months earlier while participating in any subgroup of cohort 1, or 2 months earlier while participating in cohort 2.
Inocula and challenge.
As in prior ETEC challenge studies, inocula were prepared from fresh, plate-grown organisms, using a study-specific procedure (SSP) which validated each step of the process. A dedicated laboratory room and microbiological safety cabinet were used for the inoculum preparation. Approximately 66 h before challenge, a frozen vial of ETEC H10407 was thawed and streaked onto CFA agar (8) and MacConkey agar (Becton, Dickinson and Company, Sparks, MD). The MacConkey agar plate served to further document the purity of the bacteria prior to administration to subjects. After 22 to 24 h of incubation at 35 to 37°C, a sterile cotton swab was used to touch 10 colonies from the CFA agar plate, and then the swab was mixed into a tube with 3 ml of sterile phosphate-buffered saline (PBS) (Fisher Scientific, Fair Lawn, NJ). The suspension from the PBS was used to inoculate a lawn onto 6 CFA agar plates for incubation at 35 to 37°C. After 18 to 20 h the CFA agar plates were harvested using sterile PBS, and the resulting bacterial suspension was further diluted in saline for optical density determination at 600 nm. The optical density of the suspension was adjusted to equal that corresponding to the desired concentration of bacterial cells per ml of the inoculum. The number of CFU in the inocula was determined by titrating and plating on Luria agar plates (Becton, Dickinson and Company, Sparks, MD) before and after administration to volunteers. A sample of the final inoculum was also examined by Gram stain for purity and by agglutination in anti-H10407 antiserum before being administered to subjects.
The bicarbonate buffer was prepared from USP-grade sodium bicarbonate (Fisher Scientific, Fair Lawn, NJ) by dissolving 13.35 g of sodium bicarbonate in 1,000 ml of sterile water for irrigation (Hospira, Inc., Lake Forest, IL). Subjects randomized to the sodium bicarbonate group received a total dose of 2 g of sodium bicarbonate. The CeraVacx was prepared from the commercial product (Cera Products, Inc., Columbia, MD) by dissolving 9.5 g of the powder into 150 ml of sterile water for each dose. Each dose contained 7 g of rice syrup, 2 g of sodium bicarbonate, and 0.5 g of trisodium citrate.
Volunteers were admitted to the CIR Isolation Unit the day prior to challenge. All subjects were offered a snack between 2300 and 2400 h the night prior to challenge and then not allowed any oral intake for the following ∼9 h before challenge and ∼90 min after challenge. At approximately 0900 h on the day of challenge, subjects drank 120 ml of buffer (sodium bicarbonate or CeraVacx) to neutralize gastric acidity. Approximately 1 min later, subjects drank the ETEC H10407 inoculum dissolved in 30 ml of buffer solution. In cohort 1, the addition of the bacterial inoculum was done by the research pharmacist in a manner which maintained blinding of the subjects and the investigators. The inocula were given to the subjects using opaque bottles to hide the appearance of the buffers, since they differed slightly in appearance.
Clinical evaluation of subjects.
Medical interviews and physical examinations were performed daily by the principal investigator (PI), and additional medical assessments and vital sign measurements were performed by the study team ≥3 times daily. Active solicitation regarding the following symptoms took place during the medical interview: fever, vomiting, nausea, abdominal pain, abdominal cramping, myalgias, malaise, bloating, flatulence, headache, lightheadedness, chills, constipation, and anorexia. Fever was defined as an oral temperature of ≥100.4°F. Fever severity was categorized as mild (≥100.4°F and ≤101.1°F), moderate (>101.1°F and ≤102.0°F), and severe (>102°F). Vomiting was classified as mild (1 episode within a 24-hour period), moderate (2 episodes within a 24-hour period), or severe (>2 episodes within a 24-h period). Other constitutional symptoms were graded as follows: mild (discomfort noted but no disruption of normal daily activities; relieved with or without symptomatic treatment), moderate (discomfort sufficient to reduce or affect normal daily activity; only partially relieved with symptomatic treatment), or severe (discomfort sufficient to reduce or affect normal daily activity considerably; not relieved with symptomatic treatment).
Each stool was collected using a stool collection receptacle, weighed, assessed for the presence of blood, and graded as follows: grade 1 (firm, formed), grade 2 (soft, formed), grade 3 (viscous opaque liquid or semiliquid which assumes the shape of the container), grade 4 (watery, nonviscous, opaque liquid which assumes the shape of the container), and grade 5 (clear or translucent, watery or mucoid liquid which assumes the shape of the container). Diarrhea was defined as 1 loose/liquid stool (≥grade 3) of ≥300 g or ≥2 loose/liquid stools totaling ≥200 g during any 48-hour period within 120 h of challenge with ETEC H10407. Diarrhea was classified as mild (1 to 3 diarrheal stools totaling 200 to 400 g/24 h), moderate (4 to 5 diarrheal stools or 401 to 800 g/24 h), or severe (6 or more diarrheal stools or ≥800 g/24 h).
As soon as a subject passed a diarrheal stool, oral rehydration was initiated using oral rehydration solution (Ceralyte; Cera Products, Inc., Columbia, MD) and other fluids, with the aim of replacing the subject's output. If a subject had severe vomiting, passed a large volume stool (≥300 g) at diarrhea onset, or for other reasons was unable to consume adequate amounts of oral replacement fluids to maintain hydration, intravenous (i.v.) fluids were given. Prior to discharge, a 3-day course of antibiotic therapy was initiated to clear the infection. All subjects were administered ciprofloxacin (500 mg twice daily). Trimethoprim-sulfamethoxazole (160 mg/800 mg twice daily) or amoxicillin (500 mg three times daily) was used as a backup antibiotic if ciprofloxacin could not be used. Early antibiotic treatment was provided to subjects who had severe diarrhea, moderate diarrhea for 2 days, and mild or moderate diarrhea with two or more of the following symptoms: fever (≥100.4°F), vomiting, and certain severe constitutional symptoms, including abdominal pain/cramping, headache, myalgias, or nausea. In addition, the PI could initiate early treatment if it was warranted for other reasons. Subjects who did not meet criteria for early antibiotic therapy were treated with antibiotics approximately 120 h after challenge. To be eligible for discharge, subjects needed to have at least 2 negative stool cultures for ETEC H10407. Subjects were seen as outpatients in the clinic 10 and 28 days after challenge and contacted by telephone about 3 months after challenge.
Shedding.
After challenge, all stool specimens were collected, graded, and weighed. Up to 2 samples per day were collected for fecal microbiology to detect the challenge strain. Colonization was defined as isolation of ETEC H10407 in two stool specimens collected at least 24 h after challenge.
If a subject was unable to produce at least one stool within the 24-h period, a rectal swab was obtained. Semiquantitative cultures were performed on stool samples but not on rectal swabs. Rectal swabs were placed in Cary-Blair medium and then streaked onto MacConkey agar for overnight incubation. Up to 5 colonies appearing to be E. coli were tested for agglutination with antiserum to H10407. If at least one of these colonies agglutinated, the sample was considered positive. If none of the 5 colonies agglutinated, the sample was considered negative.
For semiquantitative microbiology, the fecal sample was first diluted 10-fold up to 105 in PBS. Aliquots, consisting of 0.1 ml, of these dilutions were spread as a lawn onto MacConkey agar. After overnight incubation, the concentration of bacteria which appeared to be E. coli was calculated, and the proportion of these colonies (of 5 colonies tested) which agglutinated with anti-H10407 antisera was recorded. The anti-H10407 antiserum was prepared with rabbits at the International Centre for Diarrheal Disease Research, Bangladesh, using formalinized ETEC H10407 cells as the immunogen.
Serology.
Blood specimens were collected from volunteers in all cohorts on study days 0, 7, 10, and 28, and serum was separated to measure systemic immune responses. For cohort 3 only, blood specimens were also collected on study day 84 to measure serologic immune responses. Immunological responses to purified CFA/I (University of Gothenburg), lipopolysaccharide to the O78 antigen (LPS) (University of Gothenburg), and heat-labile enterotoxin binding subunit (LTB) (Sigma-Aldrich, St. Louis, MO) were assessed by measuring specific IgA and IgG in serum by enzyme-linked immunosorbent assay (ELISA). Secondary antibodies used were goat anti-human IgG or IgA conjugated to horseradish peroxidase (HRP) (KPL, Gaithersburg, MD). A ≥2.5-fold rise in the titer from baseline was considered a response. Study day 0 was the baseline for determining the response to ETEC H10407.
Statistical analyses.
Chi-square and t tests were used to determine differences between groups as appropriate for categorical and continuous variables. The sample size for cohort 1 was selected to provide preliminary information on approximate attack rates. With the addition of subjects in cohorts 2 and 3, the numbers of subjects receiving the selected challenge dose increased to 35. With this number of subjects and with an assumed attack rate of 70%, the 95% confidence interval for the attack rate was 55% to 85%. The sample sizes for the third cohort were selected assuming a protective efficacy rate of 90% among the rechallenged subjects and an attack rate of 70% in the naïve group. With these assumptions, there was a 90% power to detect a significant difference in attack rates between naïve and rechallenged subjects.
RESULTS
Demographics.
A total of 45 ETEC H10407-naïve subjects were enrolled in cohorts 1 to 3. Seventy-six percent of subjects were African-American, 64% of subjects were male, and the mean age was 31 years old (Table 1). Ten subjects from cohort 1 or 2 who had documented ETEC H10407-induced diarrheal illness after initial challenge were rechallenged in cohort 3. One subject enrolled in cohort 1 was challenged but was treated with antibiotics prior to any illness onset and discharged 76 h after challenge due to noncompliance with study procedures. Outpatient follow-up of this subject confirmed no clinical illness.
Table 1.
Demographics of enrolled subjects (challenged)
| Parameter | Cohort 1 |
Cohort 2 | Cohort 3 |
||||
|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | Naive | Rechallenged | ||
| No. of subjects | 5 | 5 | 5 | 5 | 15 | 10 | 10 |
| Dose given (CFU/dose) | 2 × 108 | 2 × 108 | 2 × 107 | 2 × 107 | 2 × 107 | 2 × 107 | 2 × 107 |
| Buffer | Bicarbonate | CeraVacx | Bicarbonate | CeraVacx | Bicarbonate | Bicarbonate | Bicarbonate |
| Mean age, yr (range) | 30.6 (19–45) | 28 (19–43) | 29.0 (24–41) | 33.6 (21–43) | 33.5 (19–43) | 29.1 (21–41) | 30.3 (19–43) |
| No. of females | 2 | 1 | 2 | 2 | 4 | 5 | 1 |
| No. of males | 3 | 4 | 3 | 3 | 11 | 5 | 9 |
| No. black | 3 | 3 | 4 | 4 | 11 | 9 | 7 |
| No. white | 2 | 1 | 0 | 1 | 4 | 1 | 3 |
| No. other | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Mean BMI (range)a | 30.0 (19.7–28.7) | 27.5 (23.7–33.2) | 25.6 (22.1–30.9) | 28.9 (23.0–42.0) | 27.4 (20.2–46.1) | 25.2 (20.3–29.1) | 26.1 (20.2–39.4) |
BMI, body mass index (kg/m2).
Clinical symptoms. (i) Cohort 1.
Clinical outcomes for each of the subgroups in cohort 1 are summarized in Table 2 . Diarrheal attack rates were similar across groups. Nearly all subjects who completed the ETEC challenge and ensuing follow-up developed moderate or severe diarrheal illness, regardless of dose or buffer. In addition, clinical characteristics of illness observed with each group were similar. Though the number of subjects in each group was small, the frequency of fever, early antibiotic treatment, and intravenous fluid use did not differ significantly between groups. Some subjects had unique clinical presentations. Subject number 101 who received ETEC H10407 at 2 × 107 CFU with bicarbonate (cohort 1C) had no evident illness. Subject number 120 who received ETEC H10407 at 2 × 108 CFU with CeraVacx (cohort 1B) had two distinct episodes of moderate diarrheal illness that were separated by 46 h during which time stools were normal. Upon reviewing the clinical records from cohort 1, the steering committee concluded that the volunteers in cohorts 2 and 3 should receive an inoculum dose of 2 × 107 CFU with bicarbonate buffer.
Table 2.
Clinical outcomes for subjects in cohort 1a
| Outcome | Group A (n = 5) | Group B (n = 4) | Group C (n = 5) | Group D (n = 5) |
|---|---|---|---|---|
| No. with diarrhea (%) | 5 (100) | 4 (100) | 4 (80) | 4 (80) |
| No. with moderate or severe diarrhea (%) | 4 (80) | 4 (100) | 4 (80) | 4 (80) |
| Mean [median] incubation period (hr) (range) | 43 [44] (32–51) | 42 [43] (35–48) | 43 [40] (21–69) | 57 [55] (45–71) |
| Mean [median] diarrhea stool output in 24-hour period (g) (range) | 966 [669] (383–2,418) | 765 [476] (419–1,687) | 1,032 [1,134] (350–1,511) | 666 [613] (473–966) |
| Mean [median] diarrhea total stool output (g) (range) | 1,106 [793] (719–2,418) | 1,359 [1,051] (448–2,886) | 1,707 [1,709] (1,271–2,138) | 1,021 [932] (749–1,472) |
| Mean [median] duration of diarrhea (hr) (range) | 36 [37] (12–61) | 57 [59] (4–107) | 86 [88] (23–143) | 52 [55] (28–71) |
| Mean [median] of the maximum no. of stools in 24 h (range) | 9 [6] (3–19) | 6 [6] (3–9) | 6 [6] (4–7) | 5 [5] (3–7) |
| No. of subjects with fever | 1 | 2 | 1 | 0 |
| No. of subjects with vomiting (maximum no. of vomiting episodes) | 1 (4) | 1 (4) | 1 (5) | 3 (5) |
Group A, 2 × 108 CFU with bicarbonate; group B, 2 × 108 CFU with CeraVacx; group C, 2 × 107 CFU with bicarbonate; group D, 2 × 107 CFU with CeraVacx.
(ii) Cohort 2.
The clinical outcomes for each subject in cohort 2 are shown in Table 3. Ten of 15 subjects (67%) developed moderate or severe diarrheal illness, confirming clinical trends observed with cohort 1. One additional subject (number 203) developed loose stools and constitutional symptoms consistent with ETEC-induced illness, beginning 30 h postchallenge. The subject developed severe constitutional symptoms in association with frequent loose stools and met criteria for early antibiotic therapy. However, symptoms rapidly resolved posttreatment, and the subject never met the case definition of diarrhea and was therefore not considered a case. The total volume of diarrheal purge observed with those who were cases varied widely from approximately one-third liter to nearly 6 liters.
Table 3.
Clinical outcomes for subjects in cohorts 2 and 3a
| Outcome | Cohort 2 (n = 15) | Cohort 3, naïve (n = 10) | Combined naïve subjects (n = 35) | Cohort 3, rechallenged (n = 10) |
|---|---|---|---|---|
| No. with diarrhea (%) | 11 (73) | 9 (90) | 28 (80) | 1 (10) |
| No. with moderate or severe diarrhea (%) | 10 (67) | 7 (70) | 25 (71) | 0 |
| Mean [median] incubation period (hr) (range) | 52 [47] (30–95) | 56 [54] (37–88) | 53 [49] (21–95) | 71 |
| Mean [median] maximum diarrhea stool output in 24-h period (g) (range) | 1,039 [550] (219–4,950) | 1,217 [642] (235–3,688) | 1,042 [636] (219–4,950) | 328 |
| Mean [median] diarrhea total stool output (g) (range) | 1,463 [665] (272–5,851) | 1,865 [1,021] (235–5,463) | 1,564 [1,054] (235–5,851) | 328 |
| Mean [median] duration of diarrhea (hr) (range) | 55 [72] (14–95) | 62 [73] (14–88) | 62 [72] (14–143) | 0 |
| Mean [median] of the maximum no. of stools in 24 h (range) | 8 [7] (2–18) | 5 [7] (2–16) | 7 [6] (1–18) | 1 |
| No. of subjects with fever | 3 | 3 | 6 | 0 |
| No. of subjects with vomiting (maximum no. of vomiting episodes) | 6 (3) | 5 (8) | 15 (8) | 1 (3) |
Inoculum dose and buffer were 2 × 107 CFU with bicarbonate.
(iii) Cohort 3.
Attack rates and clinical outcomes observed in ETEC H10407-naïve subjects in cohort 3 were similar to those seen with the earlier two cohorts. Nine of 10 (90%) naïve subjects developed diarrheal illness, the majority of which was classified as moderate or severe (Table 3). In contrast, one of 10 subjects rechallenged with ETEC H10407 had diarrhea which was classified as mild. This subject had a single loose stool of approximately 350 g but also vomited three times, was given i.v. fluids, and was started on early antibiotics. The remainder of subjects rechallenged with ETEC H10407 had no diarrheal illness. The difference in attack rates between naïve and rechallenged volunteers was highly significant (chi square, 9.8; P = 0.002).
Combined outcomes.
A total of 35 naïve subjects received ETEC H10407 at a dose of 2 × 107 CFU (Table 3). Twenty-eight (80%) of these subjects developed diarrhea, and the majority of this illness was classified as moderate or severe diarrhea. The mean incubation period across all groups was 53 h (range = 21 to 95 h). Mean stool outputs exceeded one liter in each group. Fever occurred in 6 of 35 subjects (17%), with a maximum recorded temperature of 101.8°F. Fifteen (43%) subjects vomited, and the maximum number of vomiting episodes was 8. Overall, 7 of 35 subjects (20%) were given intravenous fluids during the study. Among all subjects (including those who were challenged twice), 11 of 12 (92%) subjects who were given i.v. fluids had vomited, and 6 of 17 (35%) who vomited were given i.v. fluids. Twenty-one subjects (60%) were administered early antibiotic therapy, which likely had the effect of reducing both the quantity of ETEC H10407 shedding and the duration of illness. Postchallenge-solicited symptoms reported by subjects rechallenged with ETEC H10407 were remarkably diminished compared to postchallenge symptoms reported by ETEC H10407-naïve subjects, reflecting additional clinical evidence of homologous protection (Table 4).
Table 4.
Selected solicited adverse events for naïve and rechallenged subjects (107 dose)a
| AE | Combined naïve subjects (n = 35) |
Rechallenged subjects (n = 10) |
||
|---|---|---|---|---|
| Total no. | No. with moderate or severe AE | Total no. | No. with moderate or severe AE | |
| Nausea | 20 | 17 | 1 | 1 |
| Vomiting | 15 | 11 | 1 | 1 |
| Myalgia | 5 | 3 | 0 | 0 |
| Fever | 6 | 2 | 0 | 0 |
| Abdominal pain | 19 | 13 | 1 | 0 |
| Abdominal cramping | 21 | 15 | 2 | 0 |
| Malaise | 20 | 16 | 2 | 2 |
| Bloating | 15 | 9 | 0 | 0 |
| Flatulence | 27 | 11 | 7 | 0 |
| Headache | 12 | 8 | 2 | 2 |
| Lightheadedness | 11 | 10 | 1 | 1 |
| Chills | 15 | 9 | 1 | 1 |
| Anorexia | 20 | 16 | 2 | 2 |
AE, adverse event.
Microbiology.
All evaluable subjects in cohorts 1 and 2 shed ETEC H10407 in their stool postchallenge, with geometric mean maximum concentrations of approximately 2 × 108 CFU per gram in those receiving the 108-CFU dose and 8 × 107 CFU per gram in those receiving the 107-CFU dose (Table 5). Similarly, 90% of H10407-naïve subjects in cohort 3 shed the challenge organism. One ETEC H10407-naïve subject who had no diarrheal illness did not shed H10407. All but one rechallenged subject challenged in cohort 3 shed the challenge organism. Notably, the geometric mean maximum concentration in those who were rechallenged was approximately 3 × 105 CFU per gram, two logs lower than in naïve subjects (P < 0.02 versus naïve subjects) challenged with the same dose, portending protection.
Table 5.
| Time point | GMT |
||
|---|---|---|---|
| All naïve, 108 dose (n = 9) | All naïve, 107 dose (n = 35) | Rechallenged, 107 dose (n = 10) | |
| Day 2 | 1.7 × 108 | 1.5 × 107 | 7.2 × 104 |
| Maximum | 1.8 × 108 | 7.5 × 107 | 3.1 × 105 |
GMT, geometric mean titer of the number of CFU per gram of feces. For subjects who did not shed H10407, a value of 1 was used in place of 0 to calculate the GMT. If a subject had a positive qualitative sample, but negative quantitative sample, a value of 500 (corresponding to half of the lowest detectable limit of the quantitative assay) was used for the GMT calculation.
Serology.
In cohort 1, there were no significant differences in postchallenge serum immune responses between groups receiving sodium bicarbonate and CeraVacx and between groups receiving 107 and 108 doses.
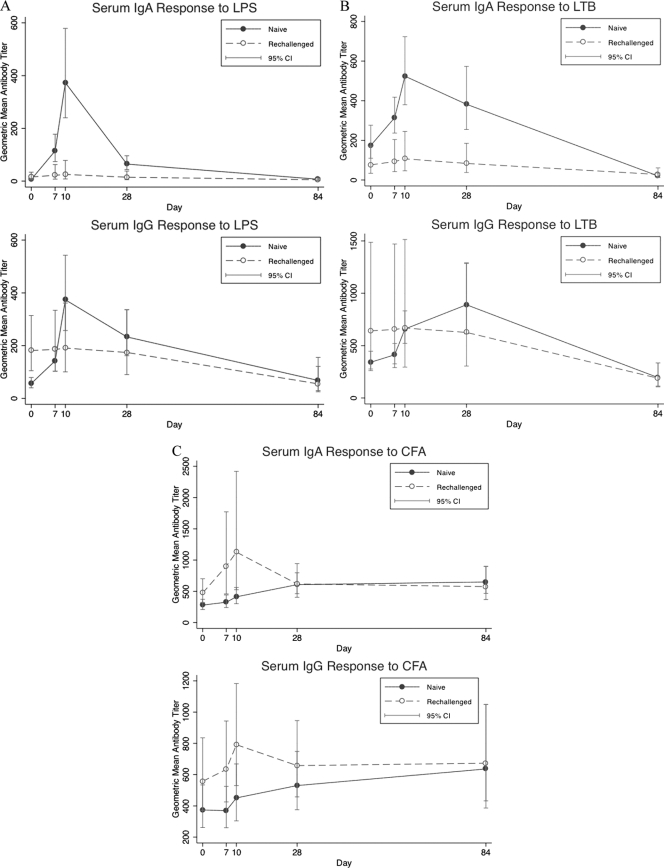
Combined serological results from ETEC-naïve recipients in all cohorts showed a dramatic increase in serum IgA and IgG immune responses to LPS O78. A total of 94% (33 of 35) and 71% (25 of 35) of subjects demonstrated an elevated IgA and IgG response to H10407 LPS, respectively (P < 0.01 for IgA and P < 0.01 for IgG). In contrast, only 30% (3 of 10) of rechallenged participants responded to LPS IgA and none responded to LPS IgG (Table 6). In both the naïve and rechallenged groups, the highest titers were observed on study day 10. The increase in the geometric mean titer (GMT) for naive volunteers was 62-fold and 7-fold higher on study day 10 for IgA and IgG, respectively, compared to baseline. The baseline GMT titer of anti-LPS IgG of rechallenged volunteers was almost 2-fold higher than the baseline GMT of naïve volunteers (Fig. 1A).
Table 6.
| Antigen | No. (%) of subjects with response |
|||
|---|---|---|---|---|
| Naïve (n = 35) |
Rechallenged (n = 10) |
|||
| IgA | IgG | IgA | IgG | |
| LPS | 33 (94) | 25 (71) | 3 (30) | 0 |
| CFA/I | 15 (43) | 8 (23) | 3 (30) | 2 (20) |
| LTB | 19 (54) | 14 (40) | 2 (20) | 0 |
A 2.5-fold or greater rise in titer from baseline was considered a response.
Fig. 1.
Serum antibody geometric mean titers (95% confidence intervals [CI]) to LPS (A), LTB (B), and CFA/I (C) following challenge with one or two doses of ETEC H10407.
Combined serological results from ETEC-naïve recipients in all cohorts showed that ≥2.5-fold rises in anti-LTB serum IgA and IgG responses developed in 54% (19 of 35) and 40% (14 of 35), respectively. In contrast, only 20% (2 of 10) of rechallenged volunteers mounted anti-LTB IgA responses and none of them developed anti-LTB IgG responses (P = 0.08 for IgA and P = 0.02 for IgG). In the ETEC-naïve group, the highest postchallenge LTB titers were seen on study day 10 for IgA and on study day 28 for IgG. As shown in Fig. 1B, LTB GMTs were 3-fold and 2.6-fold higher than baseline (study day 0), respectively.
The kinetics of the anti-CFA/I antibody rises were somewhat different than responses to LPS and LTB. The naïve and the rechallenged groups responded with similar frequencies to CFA/I. A total of 43% (15 of 35) and 23% (8 of 35) of naïve volunteers responded to CFA/I with IgA and IgG responses, respectively, while 30% (3/10) and 20% (2/10) of rechallenged volunteers responded to CFA/I with IgA and IgG responses (P = not significant). Notably, with naïve volunteers the highest CFA/I titer was observed on study day 28 in cohorts 1 and 2 and on study day 84 in cohort 3 (study day 84 was not done with cohorts 1 and 2). In contrast, the rechallenged subjects had a more rapid response, with a peak on study day 10 (Fig. 1C).
DISCUSSION
This study had three primary goals. First, we wanted to determine if a high attack rate could be achieved with a lowered inoculum dose if the challenge was preceded by an overnight fast. Second, we intended to document clinical protection against homologous rechallenge using this lowered inoculum dose. Third, we wanted to measure the serum antibody responses to a single or a repeated challenge with this strain of ETEC.
The rationale for attempting to achieve a volunteer model with a lower challenge inoculum relates to the utility of the model for ETEC vaccine evaluation. There has been concern in the field of ETEC vaccines that an excessively high inoculum dose could overwhelm vaccine-induced immunity which could otherwise be protective against natural infection. If one were to evaluate a vaccine in volunteers who were then challenged with a high dose of virulent ETEC bacteria, one might falsely conclude that the vaccine was not protective when, in fact, it might have been efficacious if tested against a lower challenge dose. The lower dose might better represent a dose confronted in “real life.”
This study demonstrated that a reproducibly high attack rate can be obtained with a dose of ETEC H10407 which is 2 logs lower than that typically used, if the challenge is preceded by an overnight fast. In previously used challenge models, the bacterial inoculum is given 90 min after breakfast and is followed by withholding food for 90 min (19). Since we did not directly compare the longer and shorter fasting periods, we cannot be certain that the longer fast leads to a higher attack rate; however, the use of the longer fast was associated with a reproducibly high attack rate with this lower dose.
It may be that the longer fasting period facilitates gastric transit or that it alters the small bowel milieu in a way that facilitates ETEC colonization. Colonization is dependent largely upon the ability of bacterial adhesins to bind to target cell receptors (10, 23). The presence of food particles, particularly simple sugars such as galactose, can inhibit ETEC colonization, and their absence could improve colonization. Indeed, previous preclinical studies showed enhanced colonization associated with longer prechallenge fasting periods (A. Svennerholm, personal communication). In addition, observational data from an earlier LT toxin challenge study suggested enhanced disease severity in subjects who fasted overnight before ingestion (D. Sack, unpublished data).
The illness observed in this study following ETEC H10407 challenge (2 × 107 CFU) was typical of ETEC diarrhea seen in previous ETEC H10407 challenge studies using higher inocula (≥108 CFU), with symptoms of watery diarrhea, episodic vomiting, and occasional mild fever (22). Though the mean purging was not as severe as is seen with volunteers challenged with V. cholerae (27), nevertheless, about 20% were given intravenous fluids and 60% were given early antibiotics. In contrast to previous studies using a higher dose, in which the mean incubation periods were typically 25 to 43 h (5, 19), the incubation period observed in this study was somewhat longer, generally exceeding 48 h. An extended incubation suggests that the lower-dose inoculum requires more time to colonize the small intestine and reach the high concentrations of bacteria sufficient to cause symptoms. Even though the time to symptom onset was longer in this study, postchallenge ETEC H10407 shedding by naïve subjects was similar in frequency and magnitude to observations in higher-dose challenge studies (3, 17, 18).
This study clearly demonstrated that subjects who experienced ETEC diarrhea earlier in the study with ETEC H10407 were protected clinically when they were exposed to a second challenge with the same strain. Homologous protection was expected since this was seen earlier in both previous animal and volunteer studies (1, 16). Nevertheless, demonstration of homologous protection with this lower-dose model was important in preparation for future vaccine studies. Protection was also observed microbiologically since the mean of the maximum concentration of H10407 in stools of rechallenged subjects was more than 2 logs lower than that in volunteers who had not been exposed previously. Though the concentrations were lower, the challenge strain was recovered from all of the volunteers who were clinically protected. This suggests that immune protection was not mediated by “sterile immunity.” Rather, targeted immunological responses likely limited the ability of the bacteria to colonize and multiply to high concentrations.
Quantitative microbiology will provide another important outcome when evaluating candidate vaccines in the future. Table 5 compares the results of the quantitative microbiology for study day 2 following challenge to the maximum numbers on any day. In fact, these results are quite similar, suggesting that future studies may choose to use results from study day 2 without monitoring quantitative microbiology every day. Specimens obtained later may be from subjects who have already been started on antibiotics, and their results would be altered by this intervention.
Volunteers challenged with H10407 had marked serological responses to known virulence antigens, including LPS, LTB, and CFA/I. Most striking was the response to LPS, an antigen which is not commonly measured in challenge studies with ETEC. Interestingly, serum antibody responses to LPS and LTB were muted following the second challenge, suggesting that the immune protection from the first challenge interfered with the response to the second. This lesser response may be due to a blocking of the colonization of the challenge strain, resulting in less exposure of the antigen to the antigen sampling cells. The response to LT is similar to that in studies of a killed oral ETEC vaccine in Egypt in which the most marked response to LT antigen occurred with the first dose, but responses to a second dose were generally not observed among those who responded to the first dose (30). In contrast to the LPS and LT responses in our volunteers, subjects who received a second challenge had an accelerated and accentuated response to CFA/I antigen, suggesting a booster response to this antigen. Since CFA/I is thought to initiate intestinal colonization, this antigen apparently did contact immune cells, and the booster response observed with the second challenge suggests that it likely takes more than a single dose to optimize the immune response to this antigen. Consistent with our findings, Evans et al. observed that persons who were previously immune to CFA mounted an anti-CFA response following oral administration of purified CFA, unlike volunteers who were not immune (9). Protective immunity is thought to be related to stimulation of local intestinal immunity, and future studies will be needed to determine the relationship between serum antibody responses and intestinal immune responses to ETEC infection.
Though the low-dose ETEC H10407 challenge model we describe here is advantageous in many respects, several limitations remain. While we were successful in maintaining a reproducibly high attack rate with a reduced challenge inoculum of 2 × 107 CFU, it is unknown whether the challenge dose could be lowered even further with this strain. In addition, it would have been ideal to directly compare these inoculum dose levels in volunteers who were fasted overnight to those with a 90-min fast, in order to establish clearly the role that a longer fast played in this model. This model used a single challenge strain which expresses LT and ST as well as CFA/I. It is unlikely that volunteer studies can be carried out with all combinations of toxin and CFA profiles. However, to facilitate development of vaccines for ETEC it would seem important to be able to demonstrate protection against ETEC diarrhea due to some additional key toxin-CFA combinations. With the rising prevalence and known pathogenicity of ETEC strains expressing ST only and CS6, the development of a relevant ETEC challenge model based on a wild-type strain containing ST with or without CS6 would be advantageous (26). Currently there are no candidate vaccines which are able to induce immunity to ST, and unless such a vaccine can be developed, vaccines will have to rely on immunity to the colonization factors or other virulence antigens.
In summary, our study documents a lower-dose volunteer model for ETEC diarrhea using strain H10407 and demonstrates that rechallenge of volunteers who had previously been ill with this strain gives protection from subsequent illness. The clinical protection is associated with a lower colonization of the pathogen, but not with sterile immunity. Serum antibody responses were demonstrated to LPS, LTB, and CFA/I, with maximum responses to LPS and LTB with a single dose observed, but a booster response was seen with CFA/I following the second dose. We anticipate that this lower-dose ETEC challenge model will be useful when evaluating ETEC vaccine candidates in the future.
ACKNOWLEDGMENTS
We thank all the volunteers who participated in this study. The steadfast efforts of Arlene Bloom, Denise Adams, Barbora Hnizda, George Gomes, and Fatuma Mawanda were invaluable. The instructive guidance of Larry Moulton and Jorge Flores is gratefully recognized. We also appreciate the contributions to protocol development and study monitoring provided by Amber Cox and Karen Charron.
Andrea Feller is supported by the Department of Health and Human Services National Institutes of Health, National Eye Institute Training grant number EY07127, Clinical Trials Training Program in Vision Research. This study was supported by PATH.
Footnotes
Published ahead of print on 18 August 2011.
REFERENCES
- 1. Ahren C. M., Svennerholm A. M. 1985. Experimental enterotoxin-induced Escherichia coli diarrhea and protection induced by previous infection with bacteria of the same adhesin or enterotoxin type. Infect. Immun. 50:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black R. E., Levine M. M., Clements M. L., Cisneros L., Daya V. 1982. Treatment of experimentally induced enterotoxigenic Escherichia coli diarrhea with trimethoprim, trimethoprim-sulfamethoxazole, or placebo. Rev. Infect. Dis. 4:540–545 [DOI] [PubMed] [Google Scholar]
- 3. Clements M. L., et al. 1981. Lactobacillus prophylaxis for diarrhea due to enterotoxigenic Escherichia coli. Antimicrob. Agents Chemother. 20:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen M. B., et al. 2002. Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect. Immun. 70:1965–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coster T. S., et al. 2007. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect. Immun. 75:252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DuPont H. L. 1995. Pathogenesis of traveler's diarrhea. Chemotherapy 41(Suppl. 1):33–39 [DOI] [PubMed] [Google Scholar]
- 7. DuPont H. L., et al. 1971. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 285:1–9 [DOI] [PubMed] [Google Scholar]
- 8. Evans D. G., Evans D. J., Jr., Tjoa W. 1977. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect. Immun. 18:330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans D. G., Graham D. Y., Evans D. J., Jr 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Response to challenge with virulent enterotoxigenic Escherichia coli.. Gastroenterology 87:934–940 [PubMed] [Google Scholar]
- 10. Evans D. G., Satterwhite T. K., Evans D. J., Jr., DuPont H. L. 1978. Differences in serological responses and excretion patterns of volunteers challenged with enterotoxigenic Escherichia coli with and without the colonization factor antigen. Infect. Immun. 19:883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans D. J., Jr, Evans D. G., Opekun A. R., Graham D. Y. 1988. Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiol. Immunol. 1:9–18 [DOI] [PubMed] [Google Scholar]
- 12. Graham D. Y., Estes M. K., Gentry L. O. 1983. Double-blind comparison of bismuth subsalicylate and placebo in the prevention and treatment of enterotoxigenic Escherichia coli-induced diarrhea in volunteers. Gastroenterology 85:1017–1022 [PubMed] [Google Scholar]
- 13. Kotloff K. L., et al. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488–1494 [DOI] [PubMed] [Google Scholar]
- 14. Levine M. M., et al. 1982. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand. J. Infect. Dis. Suppl. 33:83–95 [PubMed] [Google Scholar]
- 15. Levine M. M., et al. 1977. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect. Immun. 17:78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levine M. M., et al. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine M. M., et al. 1980. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am. J. Epidemiol. 111:347–355 [DOI] [PubMed] [Google Scholar]
- 18. McKenzie R., et al. 2007. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 25:3684–3691 [DOI] [PubMed] [Google Scholar]
- 19. McKenzie R., et al. 2008. A double-blind, placebo-controlled trial to evaluate the efficacy of PTL-003, an attenuated enterotoxigenic E. coli (ETEC) vaccine strain, in protecting against challenge with virulent ETEC. Vaccine 26:4731–4739 [DOI] [PubMed] [Google Scholar]
- 20. McKenzie R., et al. 2008. Safety and immunogenicity of WRSd1, a live attenuated Shigella dysenteriae type 1 vaccine candidate. Vaccine 26:3291–3296 [DOI] [PubMed] [Google Scholar]
- 21. Merson M. H., et al. 1976. Travelers' diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N. Engl. J. Med. 294:1299–1305 [DOI] [PubMed] [Google Scholar]
- 22. Porter C. K., et al. 2011. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29:5869–5885 [DOI] [PubMed] [Google Scholar]
- 23. Qadri F., Svennerholm A. M., Faruque A. S., Sack R. B. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sack D. A., et al. 1978. Prophylactic doxycycline for travelers' diarrhea. Results of a prospective double-blind study of Peace Corps volunteers in Kenya. N. Engl. J. Med. 298:758–763 [DOI] [PubMed] [Google Scholar]
- 25. Sack D. A., McLaughlin J. C., Sack R. B., Orskov F., Orskov I. 1977. Enterotoxigenic Escherichia coli isolated from patients at a hospital in Dacca. J. Infect. Dis. 135:275–280 [DOI] [PubMed] [Google Scholar]
- 26. Sack D. A., et al. 2007. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. Coli. diarrhoea of travellers to Guatemala and Mexico. Vaccine 25:4392–4400 [DOI] [PubMed] [Google Scholar]
- 27. Sack D. A., et al. 1998. Validation of a volunteer model of cholera with frozen bacteria as the challenge. Infect. Immun. 66:1968–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sack R. B., et al. 1971. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J. Infect. Dis. 123:378–385 [DOI] [PubMed] [Google Scholar]
- 29. Sack R. B., et al. 1975. Enterotoxigenic Escherichia-coli-associated diarrheal disease in Apache children. N. Engl. J. Med. 292:1041–1045 [DOI] [PubMed] [Google Scholar]
- 30. Savarino S. J., et al. 1998. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Egyptian adults. J. Infect. Dis. 177:796–799 [DOI] [PubMed] [Google Scholar]
- 31. Svennerholm A. M., Tobias J. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev. Vaccines 7:795–804 [DOI] [PubMed] [Google Scholar]
- 32. Tacket C. O., et al. 1992. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am. J. Trop. Med. Hyg. 47:276–283 [DOI] [PubMed] [Google Scholar]
- 33. Tribble D. R., et al. 2009. Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin. Infect. Dis. 49:1512–1519 [DOI] [PubMed] [Google Scholar]
- 34. Walker R. I., Steele D., Aguado T. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545–2566 [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization. 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly. Epidemiol. Rec. 81:97–104 [PubMed] [Google Scholar]