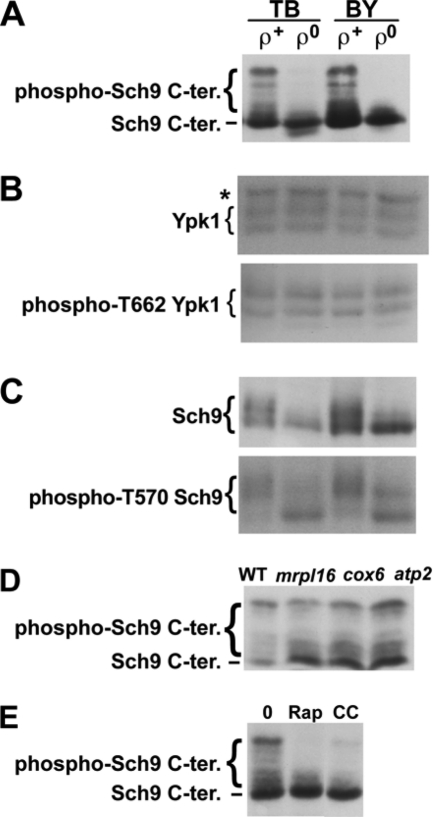
Fig. 1.
Phosphorylation of a TORC1 and Pkh1/2-target (Sch9) and a TORC2 target (Ypk1). (A) TORC1-dependent Sch9 phosphorylation in ρ+ and ρ0 cells carrying pJU676 and pJU450 in TB50 (TB [strain RL170-2b]) and BY4742 (BY) backgrounds. Prior to SDS-PAGE/anti-HA blot, protein extracts were treated with NTCB to cleave proteins. (B) Total Ypk1 (upper panel, anti-Ypk1 blot) and phospho-T662 Ypk1 (lower panel, anti-phospho-T659 Ypk2 blot) in uncleaved protein extracts derived from the cells shown in panel A. The band marked with an asterisk (*) represents a non-Ypk1 protein that cross-reacts with the anti-Ypk1 antibody. (C) Total Sch9-HA (upper panel, anti-HA blot) and phospho-T570 Sch9-HA (lower panel, anti-phospho-T570 Sch9 blot) analyzed in uncleaved extracts as described for panel B. Phosphorylation-dependent migration shifts are clearly visible in the upper panel, but these shifts are not due to changes in T570 phosphorylation status (lower panel). (D and E) TORC1-dependent Sch9 phosphorylation, as determined by anti-HA Western blotting of NTCB-treated protein extracts obtained from WT and pet cells (D) and ρ+ cells (YGSK226) (E) after treatment with 200 ng/ml rapamycin (Rap) or 4 μg/ml CCCP (CC) or drug vehicle (0). All treatments were for 70 min. Stocks (1,000×, 200 μg/ml rapamycin and 4 mg/ml CCCP) were prepared in ethanol-Tween 20 (9:1).