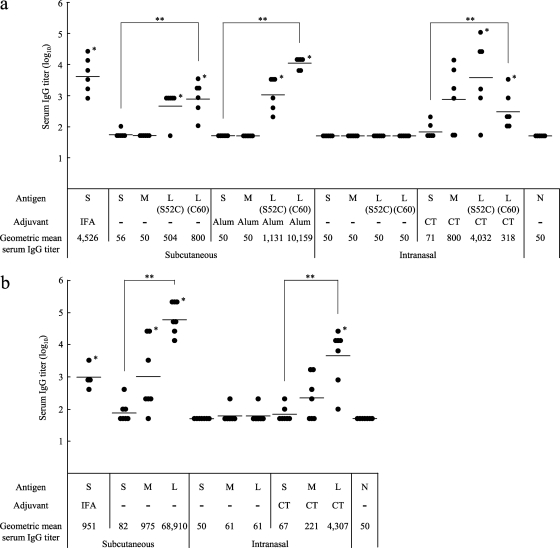
Fig. 4.
Immunogenicity of the tricomponent complex. Mice were immunized by the subcutaneous or intranasal route three times, at weeks 0, 2, and 4, and antisera were collected 2 weeks after the third immunization to evaluate the Pvs25-specific IgG titers. All mice received 30 μg of the Pvs25H-A antigen as a conjugated or unconjugated protein. Incomplete Freund's adjuvant (IFA), aluminum hydroxide (Alum), or cholera toxin (CT) (1 μg) was used as the subcutaneous or intranasal adjuvant, as indicated. N, nonimmune sera from unimmunized control mice. Antibody titers were defined either as the serum dilution that resulted in an OD415 of 0.1 or as the serum dilution for which a 1-point-higher dilution (2-fold) resulted in an OD415 of <0.1. (a) Female BALB/c mice (six per group) were immunized with either the Pvs25H-A antigen alone (30 μg) (S), a mixture of the antigen (30 μg) and the TB-Z (21.4 μg) (M), the TB(S52C)-Z:Pvs25H-A tricomponent complex (51.4 μg) [L(S52C)], or the TB(C60)-Z:Pvs25H-A tricomponent complex (51.4 μg) [L(C60)]. (b) Female BALB/c mice (four or seven per group) were immunized with either the Pvs25H-A antigen alone (30 μg) (S), a mixture of the antigen (30 μg) and the COMP-Z (10.8 μg) (M), or the COMP-Z:Pvs25H-A tricomponent complex (40.8 μg) (L). Asterisks indicate significant differences from the unimmunized control group by the Wilcoxon-Mann-Whitney test (*, P < 0.05) or among the three groups indicated [S, M, and L(S52C) or L(C60) for the TB-based constructs, or S, M, and L for the COMP-based constructs] by the Kruskal-Wallis test (**, P < 0.001).