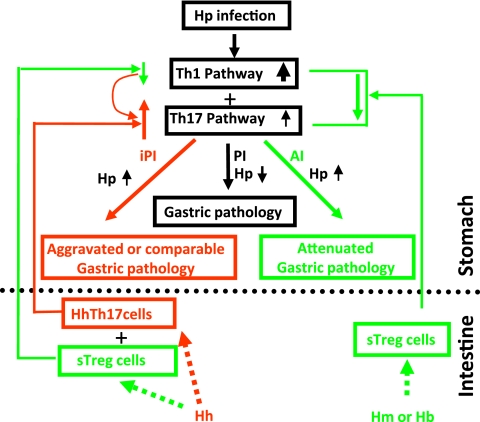
Fig. 7.
Proposed working model underlying EHS-dependent effects on H. pylori-induced gastric pathology in C57BL/6 mice. H. muridarum/H. bilis-sensitized TREG (sTREG) cells with anti-inflammatory properties migrate to the stomach, where they dampen host proinflammatory responses to subsequent H. pylori infection by decreasing expression of proinflammatory cytokines, including Ifn-γ, Tnf-α, and Il-1β, as well as Il-17A. This downregulation, despite higher colonization levels of gastric H. pylori, attenuates H. pylori-induced gastritis. In contrast, H. hepaticus may be less potent in sensitizing TREG cells and more potent in stimulating Th17 cells (HhTh17), which then also migrate to the stomach upon H. pylori infection. H. hepaticus sTREG cells may moderately downregulate proinflammatory Th1 cytokines, such as Ifn-γ, which play an important role in helicobacter clearance (40), thereby permitting higher colonization levels of H. pylori in HhHp mice than in mice infected with H. pylori only. H. hepaticus Th17 cells in addition to relatively lower levels of Ifn-γ would markedly increase signals of the Th17 pathway and thereby lead to a more severe H. pylori-induced gastric pathology in HhHp mice than in mono-H. pylori-infected mice. Our data show that alteration of these H. pylori-induced proinflammatory cytokines by coinfection with an EHS was more evident at 6 MPI than at 11 MPI, indicating that the interplay among the Th1, TREG, and Th17 pathways occurred early in infection, potentially prior to development of overt gastritis. iPI, EHS-induced proinflammatory responses; PI, proinflammatory responses; AI, anti-inflammatory responses. Upward and downward arrows depict up- and downregulation of associated pathways compared to mono-H. pylori infection, respectively.