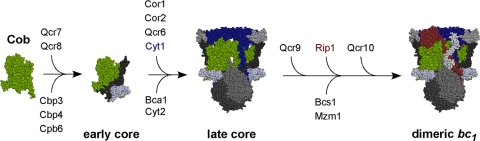
Fig. 1.
Schematic for assembly of the bc1 complex in yeast mitochondria. The Cob translational activator Cbp6 and assembly factor Cbp3 associate with the nascent polypeptide as it exits the mitochondrial ribosome. Cbp3 and Cbp6 remain bound to the newly synthesized Cob, and Cbp4 is recruited to this complex. The supernumerary subunits Qcr7 and Qcr8 associate with Cob (green) to form the early core complex. The core protein subunits, Cor1 and Cor2, along with Qcr6 subunits and the catalytic subunits of Cyt1 (blue), are added to form the late core complex. Bca1 is a recently identified assembly factor for bc1 that acts prior to formation of the late core, and Cyt2 is the heme lyase. The assembly factors Bcs1 and Mzm1 assist in formation of the functional homodimeric bc1 complex by assisting in the insertion of Rip1 (shown in ruby). Qcr9 and Qcr10 subunits are also added at a late step. Protein subunits of the bc1 enzyme complex are listed above the arrows; nonsubunit proteins are listed below.