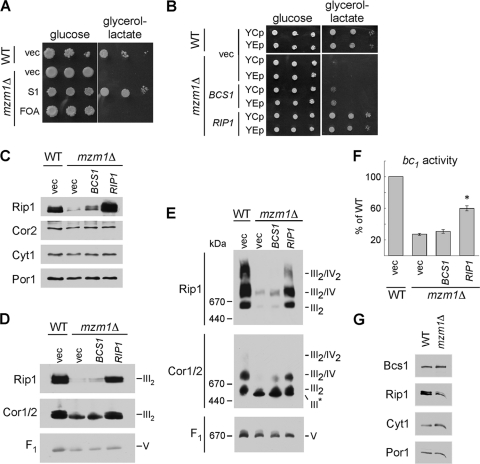
Fig. 3.
Suppression of the 37°C growth defect and rescue of the reduced Rip1 levels, supercomplex formation, and bc1 complex activity of mzm1Δ. (A) Growth test at 37°C on glucose or glycerol-lactate medium of mzm1Δ cells transformed with vector control (vec) or a plasmid isolated from the suppression screen (S1), which was identified by sequencing to contain RIP1. Loss of this RIP1-containing plasmid was induced by 5-fluoroorotic acid treatment (FOA). (B) Growth test at 37°C on glucose or glycerol-lactate medium of mzm1Δ cells transformed with low-copy-number (YCp) and high-copy-number (YEp) vector control or plasmids containing BCS1 or RIP1. (C to E) SDS-PAGE (C) and BN-PAGE immunoblots with either 1% dodecylmaltoside solubilization (D) or 1% digitonin solubilization (E) of mitochondria from mzm1Δ cells transformed with YEp vector control or plasmids containing BCS1 or RIP1. The late core subassembly intermediate is designated by III*. (F) bc1 complex activity of mitochondria from mzm1Δ cells transformed with high-copy-number vector control or plasmids containing BCS1 or RIP1, shown as a percentage of WT activity, with the asterisk indicating significantly increased activity relative to mzm1Δ with vector control (n = 6 ± standard deviation). Statistical significance was determined by analysis of variance with Bonferroni's post hoc test in Kaleidagraph. (G) SDS-PAGE immunoblot of mitochondria from Bcs1::Myc cultures with and without Mzm1. Bcs1 protein levels were identified using anti-Myc antibody.