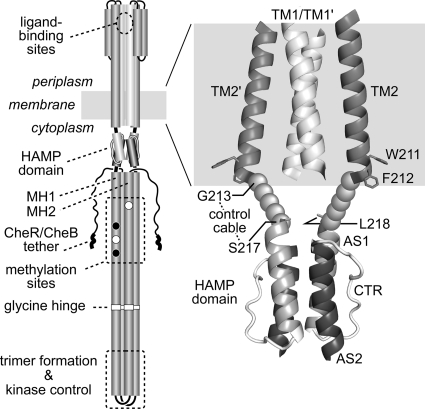
Fig. 1.
Tsr structure and location of the control cable. (Left) The native Tsr molecule is a homodimer of 551-residue subunits. Cylindrical segments represent α-helical regions, drawn approximately to scale. Methylation sites shown as black circles indicate glutamine residues that must be deamidated to glutamates by CheB before they can accept methyl groups, and open circles represent glutamate residues that are direct substrates for the CheR methyltransferase. Thickened regions at the C terminus of each subunit represent a pentapeptide sequence (NWETF) to which CheB and CheR bind. (Right) Expanded view of the TM2-HAMP region of Tsr. The structure of the 4-helix TM bundle is based on modeled coordinates for the TM bundle of the related receptors Trg and Tar (48). Side chains for aromatic residues W211 and F212 are shown as stick representations and mark the cytoplasmic end of TM2. The HAMP bundle is modeled on the structural coordinates for Af1503 HAMP (28). L218 is the first functionally critical residue of the Tsr HAMP bundle (74). The structure of the control cable, defined as the segment from G213 to S217, is unknown.