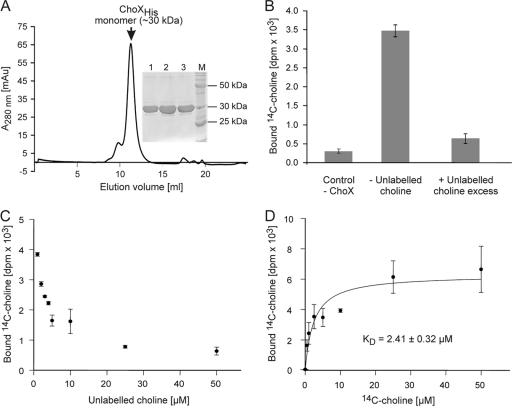
Fig. 5.
Purification and in vitro choline-binding properties of recombinant ChoX. (A) Size exclusion chromatography of Ni-IDA-purified PmtA. The peak fractions 1, 2, and 3 were analyzed by SDS-PAGE as depicted on the right. AU, absorbance units. Lane M (inset), BenchMark protein standard (Invitrogen). (B) Radioactive choline-binding activity of recombinant ChoX. Choline-binding activity was analyzed with 5 μM ChoX and 5 μM [14C]choline over 5 min. The displacement assay (+unlabeled choline excess) contained, in addition, 10-fold unlabeled choline. (C) Competitive displacement of radioactively labeled choline by unlabeled choline (0 to 50 μM). The assay mixture contained 5 μM recombinant ChoX and 5 μM [14C]choline. (D) Plots of choline-binding data for wild-type ChoX. A reaction volume of 50 μl contained 5 μM recombinant ChoX and 0 to 50 μM [14C]choline. Changes in bound [14C]choline (y axis) were plotted against choline concentration (x axis). All data sets were fitted to the equation for one-site binding (see Materials and Methods) by nonlinear regression using SigmaPlot, version 9.0. The points shown are the mean values of three individual experiments.