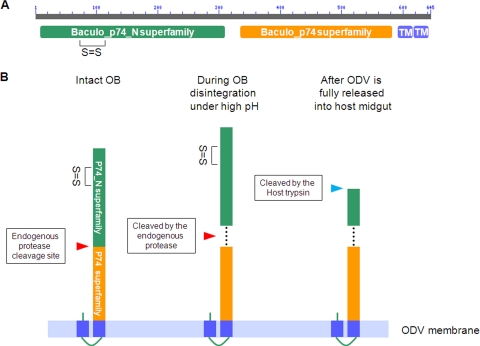
Fig. 8.
Proposed model for sequential cleavage of P74 in L-ODV based on conserved domain predictions and experimental data. (A) Conserved domains in P74. The AcMNPV P74 amino acid sequence was analyzed using the NCBI Conserved Domains server. Baculo_p74_N (green block) and Baculo_p74 superfamilies (orange block) were identified, as indicated. The homology with the Baculo_p74_N superfamily starts with amino acid (aa) 5 and lasts to aa 309 of AcMNPV P74. The Baculo_p74 superfamily domain starts at aa 333 and lasts until aa 583. The only disulfide bond (between cysteine 72 and cysteine 109) predicted with high probability by the DiANNA server is indicated. The score for this prediction was 0.99261, within a range of 0 to 1. Two conserved C-terminal transmembrane domains predicted by the TMHMM 2.0 server are also indicated (TM; blue blocks). (B) Model of P74 sequential cleavage. The two conserved P74 domains and the potential intramolecular disulfide bond in the P74-N subunit are indicated. The first cleavage of P74 is proposed to happen between the two conserved domains and during OB disintegration under alkaline conditions. A potential mode of interaction between the two fragments is indicated by dashed lines. After the first cleavage by the endogenous protease, the P74-N subunit is predicted to undergo a second proteolytic cleavage by a host trypsin present in the insect midgut.