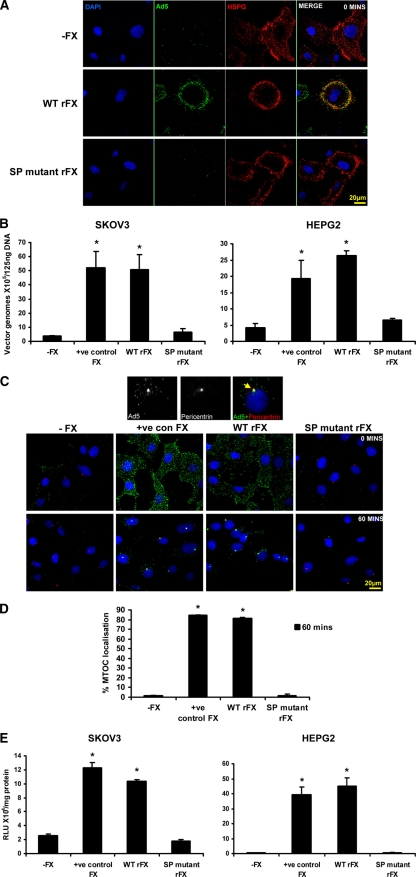
Fig. 3.
Critical role of the heparin binding proexosite in the FX SP domain in Ad5 cell binding and gene transfer in vitro. (A) Alexa 488-labeled Ad5 (green) at 10,000 vp/cell was allowed to bind SKOV3 cells for 1 h at 4°C in the absence of FX or the presence of WT rFX or SP mutant rFX. Cells were then stained with a pan-heparan sulfate antibody (clone 10E4) (red). Nuclei were counterstained using DAPI. Images were captured on a confocal microscope at 63× objective. (B) Binding of Ad5 at 1,000 vp/cell to SKOV3 cells or Ad5KO1 at 1,000 vp/cell to HepG2 cells for 1 h at 4°C in the presence or absence of pd FX (positive control FX), WT rFX, or SP mutant rFX was quantified. Vector genomes were detected by quantitative SYBR green PCR. (C) Alexa 488-labeled Ad5 (green) at 10,000 vp/cell was allowed to bind SKOV3 cells for 1 h at 4°C in the absence or presence of pd FX, WT rFX, or SP mutant rFX. Cells were then incubated at 37°C for 0 or 60 min prior to fixation and staining for the MTOC marker pericentrin (red). Nuclei were counterstained using DAPI. Images were captured on a confocal microscope at 63× objective. (D) The percentage of cells with colocalization of fluorescently labeled Ad5 with the MTOC marker pericentrin was calculated by analyzing at least five separate 40× microscope fields per experimental condition. (E) SKOV3 and HepG2 cell transduction at 48 h postinfection after a 3-h exposure to Ad5 or Ad5KO1 in the presence or absence of pd FX, WT rFX, or SP mutant rFX conditions. RLU, relative light units. Error bars represent standard errors of the means.