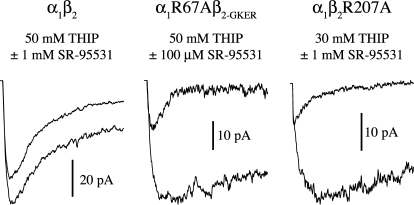
Fig. 4.
The mutations β2R207A and α1R67A slow the binding rate of THIP. Responses to simultaneous application of THIP and the competitive antagonist SR-95531 reflect their relative binding rates. Pulses were 500 ms and the first 100 ms are shown. The larger amplitude traces are responses to THIP alone, whereas the smaller traces are responses to coapplication of THIP and antagonist. The ratio of the peak currents was used to compute kon-THIP (Jones et al., 1998).