Abstract
Atlas Genetics has developed a Point-of-Care device for Chlamydia trachomatis utilizing a novel electrochemical detection principle. The assay has a time-to-result of less than 25 minutes. An independent pre-clinical validation study using 306 pre-typed clinical samples determined a clinical sensitivity of 98.1% and specificity of 98.0%.
Index Terms: Point-of-Care, Chlamydia, infectious disease, electrochemistry
I. INTRODUCTION
Chlamydia trachomatis infection is one of the most common sexually-transmitted diseases (STDs), causing an estimated 92 million new cases of genital Chlamydia infection every year [1]. Chlamydia is a bacterial infection that can be cured with antibiotics. However, many patients experience no symptoms and often go undiagnosed and untreated, which may lead to severe health consequences, especially for women where at least 70% of Chlamydia infections are asymptomatic [2]. If left untreated in women, these infections can result in pelvic inflammatory disease, causing long-term complications such as chronic pelvic pain, ectopic pregnancy, and infertility [3]. In the US, untreated STDs are estimated by the Centers for Disease Control (CDC) to cause at least 24,000 women to become infertile each year. Although serious health consequences are less common among men, untreated Chlamydia infection can cause epididymitis, urethritis, and in rare cases, sterility [4]. Early diagnosis and treatment are essential, but many patients do not attend specialist Genitourinary Medicine (GUM) clinics for testing due to the asymptomatic nature of the infection and the stigma associated with these clinics. In an attempt to address these problems, government-sponsored programs have been established in the UK, US and elsewhere which aim to increase testing by promoting opportunistic screening at a variety of new venues such as family planning and other primary healthcare clinics [5]. While screening promotes increased testing, result turnaround times of 5–10 days and the frequent failure of patients to attend follow-up appointments (drop-out rates of up to 50% are frequent in some US public health clinics) combine to be a major factor in preventing effective treatment. Such delays in treatment also contribute to the spread of the disease. An accurate, reliable and rapid Point-of-Care (PoC) test for Chlamydia would enable a patient to be tested and treated in a single short visit, whether this be at the GUM or family planning clinic, doctor’s office or pharmacy. To meet the need for a rapid PoC diagnostic test for Chlamydia, Atlas Genetics is developing Velox™, a platform technology capable of running both molecular and immunoassays with a time-to-result of under 25 minutes.
II. TECHNOLOGY
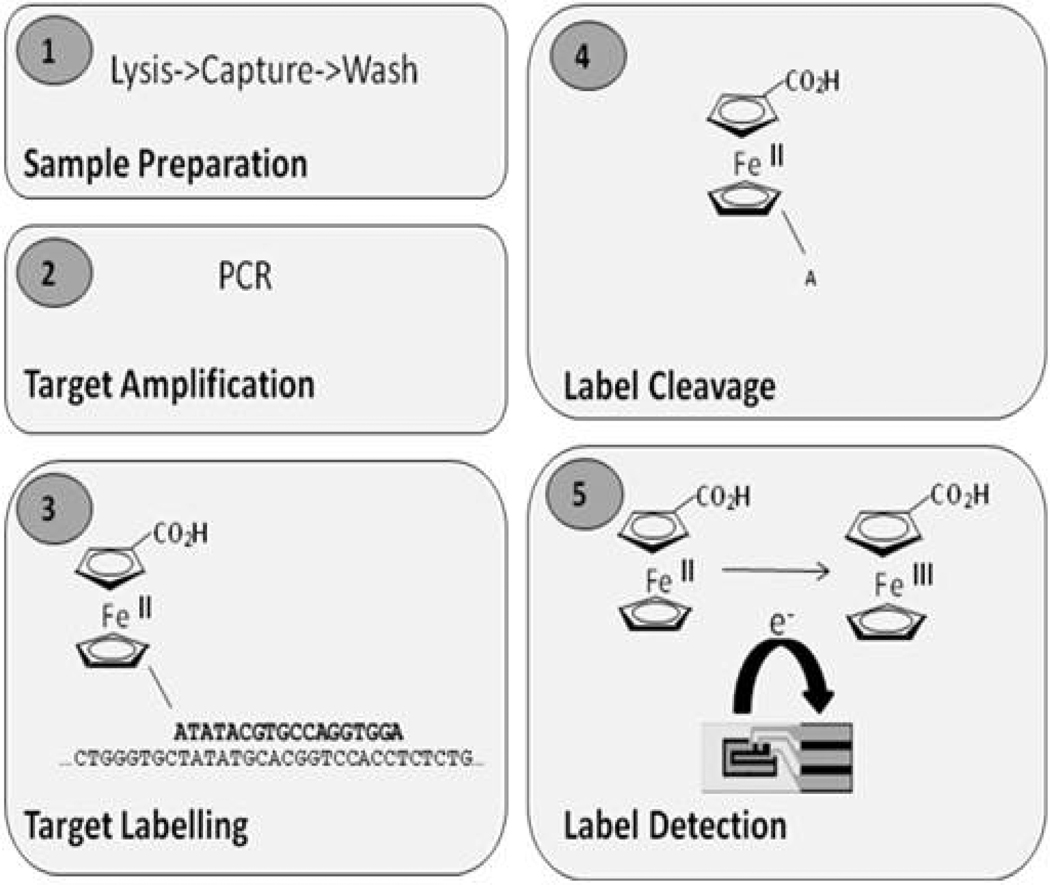
The Velox™ technology comprises a fully-integrated fluidic card for sample processing and reagent handling and incorporates a novel technique for detection of our proprietary electrochemical labels. The test card will be processed and analysed using a low-cost reader instrument that provides fluidic and temperature control, end-point electronics and software. This system is capable of processing both molecular and immunoassay tests. The integrated card contains all reagents necessary to run a test (both wet and dried reagents) and eliminates all complicated user steps, making the system applicable to PoC environments. The Chlamydia assay comprises a number of steps that include extraction of Chlamydial DNA from a clinical sample, specific amplification of a small segment of the DNA sequence by Polymerase Chain Reaction (PCR) and then sensitive and specific detection of the amplified DNA using an electrochemically-labeled DNA probe. Figure 1 shows a schematic of the molecular assay. To date, development has been focused on detection of DNA sequences using electrochemical labels based on ferrocene derivatives, described in references [6–8]. The standard oxidation state of ferrocene is +2, and it undergoes a one-electron oxidation to ferricenium at a low oxidation potential (Figure 1, panel 5). It has a number of advantages as a label, including a well-characterized redox behavior, low oxidation potential and easily-synthesized derivatives. A number of different ferrocene labels have been synthesized, including both mono-and di-ferrocenes. Oligonucleotide probes can be labeled with any one of a number of ferrocene derivatives, each with its own electrochemical property. This provides a simple strategy for the simultaneous analysis of a single clinical sample for the presence of multiple pathogens. The steps for processing a molecular test are as follows. Clinical sample (e.g. swab eluate, urine) is introduced by the user into the sample inlet on the analyte-specific test card. This is the only step that involves user interaction; the following steps are fully automated. The sample is processed by extracting the nucleic acid from the sample, which is immobilized on an adsorbent matrix. The extracted DNA is washed to remove contaminants (such as cellular debris, proteins and mucus), and is air-dried. The purified DNA is eluted from the matrix and is pumped to the amplification chamber that contains all the dried reagents necessary for PCR. The instrument’s thermal control system combined with the unique design of the card’s PCR chamber allows efficient, rapid thermal cycling to take place. Following target DNA amplification the PCR product is transported to the electrochemical detection chamber where a short, single-stranded DNA probe complementary in sequence to the target Chlamydial DNA and conjugated to a ferrocene label is hybridized to the amplicon (Figure 1, panel 3). The probe/target DNA hybrid is incubated with a double-strand specific exonuclease which allows cleavage of the terminal nucleotide (Figure 1, panel 4). The liberated mononucleotide-ferrocene label is detected in-situ on the surface of a screen-printed electrode (Figure 1, panel 5) using differential pulse voltammetry (reviewed in [9]). The single nucleotide coupled to the ferrocene moiety is preferentially detected on the electrode due to mass diffusion and surface adsorption constraints on the full-length probe.
Fig. 1.
Molecular assay – schematic.
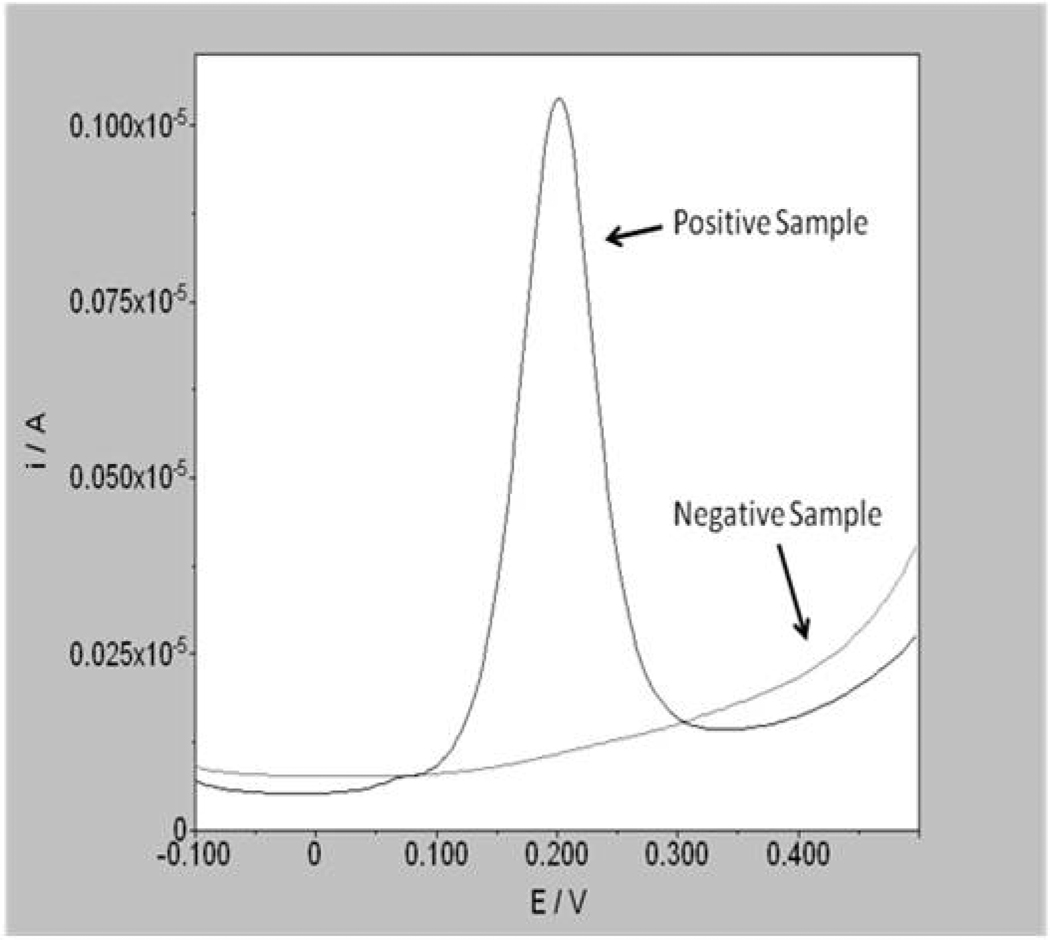
Where the ferrocene label has been cleaved from the probe/target complex (i.e. a sample positive for Chlamydia) a peak in current is observed at a voltage which corresponds to the oxidation potential of the label. Where no ferrocene label is released (i.e. a sample negative for Chlamydia), no peak is observed (Figure 2). The assay takes approximately 25 minutes to complete from the point of sample addition. Although there are many available DNA detection systems for nucleic acid targets, for application in PoC settings, tests need to be rapid (<30 minutes), simple to use, multiplex and low cost. Many of the Nucleic Acid Amplification Tests (NAATs) currently available utilize fluorescent detection methods which significantly increase the cost of the reader instrument required for data acquisition. The instrumentation requirement for electrochemical analysis is significantly simpler, and cheaper, than for fluorescence detection which makes it more robust and transportable to different environments. Electrochemical detection has already gained acceptance in the PoC market through the widespread use of the technology in blood glucose meters. Both the instrumentation and tests are easily miniaturized which is an advantage for both laboratories and PoC environments where space for the instrument, and the storage of disposables, is at a premium. In contrast to fluorescent detection methods optical clarity is not required, which makes for simpler sample processing and fewer restrictions on the materials that can be used in the device. DNA probes for different analytes can be labeled with a number of ferrocene derivatives with different redox potentials enabling multiplex assays. It is envisaged that the test menu will be increased from Chlamydia to other STDs, including both molecular and immunoassay targets.
Fig. 2.
Detection of positive and negative samples using differential pulse voltammetry.
III. EXPERIMENTAL
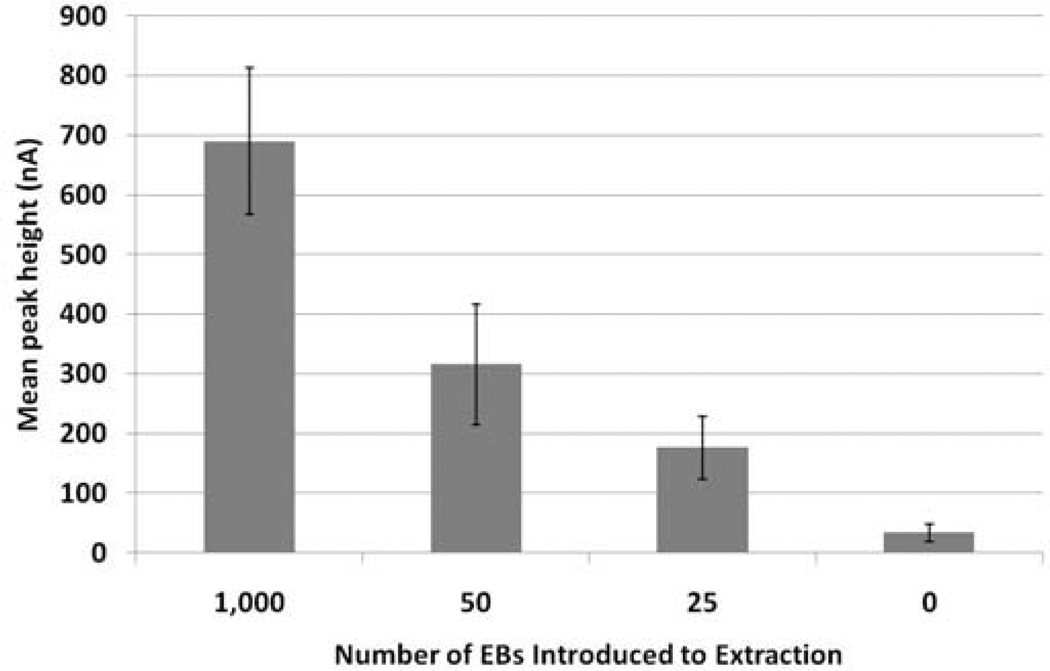
A bench-top (laboratory) version of the assay for Chlamydia has been fully developed and tested using both Chlamydia cells (in the form of Elementary Bodies (EBs)) and clinical samples. This enabled both analytical sensitivity (Limit of Detection (LoD)) and specificity to be determined. Analytical sensitivity was determined by adding 1,000, 50, 25 and 0 EBs to aliquots of swab transport media. DNA was extracted from the cells which was subsequently amplified and detected using the electrochemical method described above. Figure 3 shows the LoD data. The assay is extremely sensitive with an LoD of 25 EBs and exhibits very low background.
Fig. 3.
Analytical sensitivity (LoD) of the Chlamydia bench-top assay. Error bars show the standard deviation (n=6).
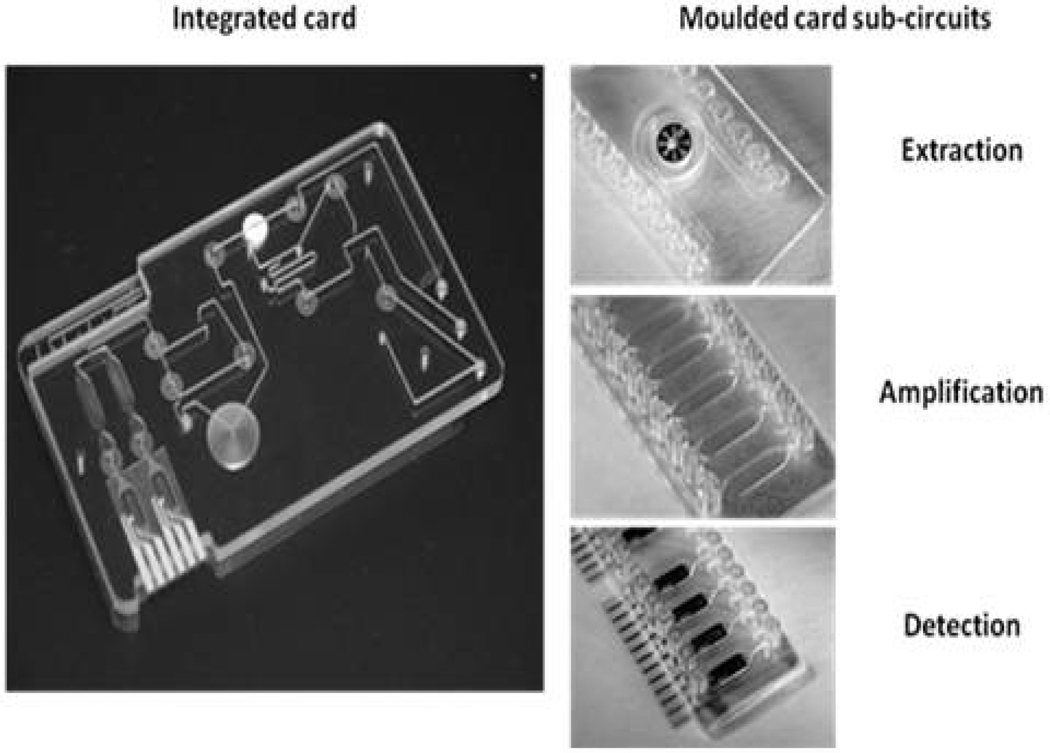
The assay detects all 15 C. trachomatis serovars and does not cross-react with human DNA nor DNA from a panel of 85 microorganisms selected as either likely to be found in human genital swab samples, or closely genetically related to C. trachomatis. Clinical sensitivity and specificity was determined using 102 discard Chlamydia swab samples (52 positive, 50 negative), previously identified using the BD ProbeTec™ ET system, an existing NAAT used routinely for the screening and diagnosis of Chlamydia. These samples were tested using the bench-top assay and showed a clinical sensitivity of 96% and specificity of 98%. Prior to full transfer of the complete assay on to the integrated test card, prototypes of each of the three assay sub-circuits were produced, one for each of the three assay steps (extraction, amplification and detection, see Figure 4). This approach will enable each assay component to be thoroughly optimised prior to full integration and included the use of dried reagents formulated to work with the final materials and fluidic geometries.
Fig. 4.
Prototype integrated test card and individual moulded card sub-circuits used in assay development. The molded card sub-circuits allowed the three individual stages of the assay to be optimized prior to selection of final plastics, geometries and reagent formulations to be used in the integrated card.
Although all assay sub-circuits are being tested in this manner, the following work describes an independent preclinical study of the electrochemical detection sub-circuit by the Center for Point-of-Care Tests for Sexually Transmitted Diseases at Johns Hopkins University (JHU) School of Medicine. JHU were provided with sufficient electrochemical detection sub-circuit modules (see Figure 4) containing both dried electrochemical probe and exonuclease to run 306 clinical samples that had been pre-typed as positive (n=107) or negative (n=199) for Chlamydia using either the Gen-Probe Aptima Combo 2™ or the Roche Amplicor™ C. trachomatis NAATs. For testing using the detection sub-circuit, the clinical samples were extracted using the Roche MagNA Pure DNA extraction system and amplified using the bench-top PCR protocol prior to on-card detection. This independent study showed a clinical sensitivity of 98.1% and a specificity of 98.0% (Table 1), demonstrating the applicability of using electrochemical detection in PoC settings.
TABLE I.
Results of the detection sub-circuit pre-clinical validation study.
| Total (Roche & Gen-Probe) |
Comparator Status | ||
|---|---|---|---|
| Negative | Positive | ||
|
Atlas Results |
Negative | 195 | 2 |
| Positive | 4 | 105 | |
| Sensitivity (%) | 98.1 | ||
| Specificity (%) | 98.0 | ||
IV. CONCLUSIONS
We have designed a highly sensitive and specific assay for Chlamydia on a PoC system that is capable of processing both molecular and immunoassay-based tests. The applicability of the bench-top electrochemical assay was demonstrated initially using pre-typed clinical samples. An independent validation carried out to test the fluidic detection sub-circuits demonstrated a clinical sensitivity and specificity of 98.1% and 98.0%, respectively. The technology described above is being developed for use in PoC testing and the performance characteristics obtained correlate well with those for existing NAATs which are typically reported as having sensitivities and specificities of between 90–95% and 98–100%, respectively, but have assay times-to-result of around four hours [10]. The next stage of development will be to reduce the time required for target amplification on-card. Initial experimentation indicates a time-to-result of around 25 minutes using the Velox™ platform for a molecular assay. The method of electrochemical detection described here is well suited to a PoC application and the fact that many electrochemical labels are available provides a simple strategy for the simultaneous analysis of a single clinical sample for the presence of multiple pathogens.
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health under Grant U54 EB007958.
Footnotes
Personal use of this material is permitted. However, permission to use this material for any other purposes must be obtained from the IEEE by sending an pubs-permissions@ieee.org.
Contributor Information
David M. Pearce, Atlas Genetics Ltd., Oak House, White Horse Business Park, Trowbridge, Wiltshire, BA14 0XG, UK david.pearce@atlasgenetics.com
Daniel P. Shenton, Atlas Genetics Ltd., Oak House, White Horse Business Park, Trowbridge, Wiltshire, BA14 0XG, UK dan.shenton@atlasgenetics.com
Jeffrey Holden, Division of Infectious Diseases, Johns Hopkins University School of Medicine, 530 Rangos Building, 855 North Wolfe Street, Baltimore, MD 21205, USA jholden1@jhu.edu.
Charlotte A. Gaydos, Division of Infectious Diseases, Johns Hopkins University School of Medicine, 530 Rangos Building, 855 North Wolfe Street, Baltimore, MD 21205, USA cgaydos@jhmi.edu
REFERENCES
- 1.WHO. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. Geneva: WHO; 2001. [PubMed] [Google Scholar]
- 2.The UK Collaborative Group for STI and HIV Surveillance. Testing Times – HIV and other Sexually Transmitted Infections in the United Kingdom. London: Health Protection Agency; 2007. pp. 44–49. [Google Scholar]
- 3.Hillis SD, Wasserheit JN. Screening for Chlamydia – a key to the prevention of pelvic inflammatory disease. N Engl. J. Med. 1996 May;vol. 334(no. 21):1399–1401. doi: 10.1056/NEJM199605233342111. [DOI] [PubMed] [Google Scholar]
- 4.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling PF, Stamm WE, editors. Sexually Transmitted Diseases. 4th ed. New York: McGraw-Hill; 2008. pp. 575–593. [Google Scholar]
- 5.National Chlamydia Screening Programme (2010) [Online]. Available: http://www.chlamydiascreening.nhs.uk/
- 6.Hillier SC, Frost CG, Jenkins ATA, Braven HT, Keay RW, Flower SE, Clarkson JM. An electrochemical study of enzymatic oligonucleotide digestion. Bioelectrochem. 2004;vol. 63:307–310. doi: 10.1016/j.bioelechem.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Hillier SC, Flower SE, Frost CG, Jenkins ATA, Keay R, Braven H, Clarkson J. An electrochemical gene detection assay utilizing T7 exonuclease activity on complementary probe-target oligonucleotide sequences. Electrochem. Commun. 2004;vol. 6:1227–1232. [Google Scholar]
- 8.Muir A, Jenkins ATA, Forrest G, Clarkson J, Wheals A. Rapid electrochemical identification of pathogenic Candida species. J. Med. Microbiol. 2009 Sep.vol. 58:1182–1189. doi: 10.1099/jmm.0.009183-0. [DOI] [PubMed] [Google Scholar]
- 9.Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd ed. Hoboken, NJ: John Wiley; 2001. pp. 286–303. [Google Scholar]
- 10.Herring A, Ballard R, Mabey D, Peeling R. WHO/TDR Sexually Transmitted Diseases Diagnostics Initiative, Evaluation of rapid diagnostics tests: chlamydia and gonorrhoea. Nature Rev. Microbiol. (Suppl.) 2006 Dec.vol. 4(no. 12):S41–S48. doi: 10.1038/nrmicro1562. [DOI] [PubMed] [Google Scholar]