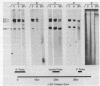
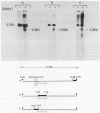
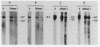Abstract
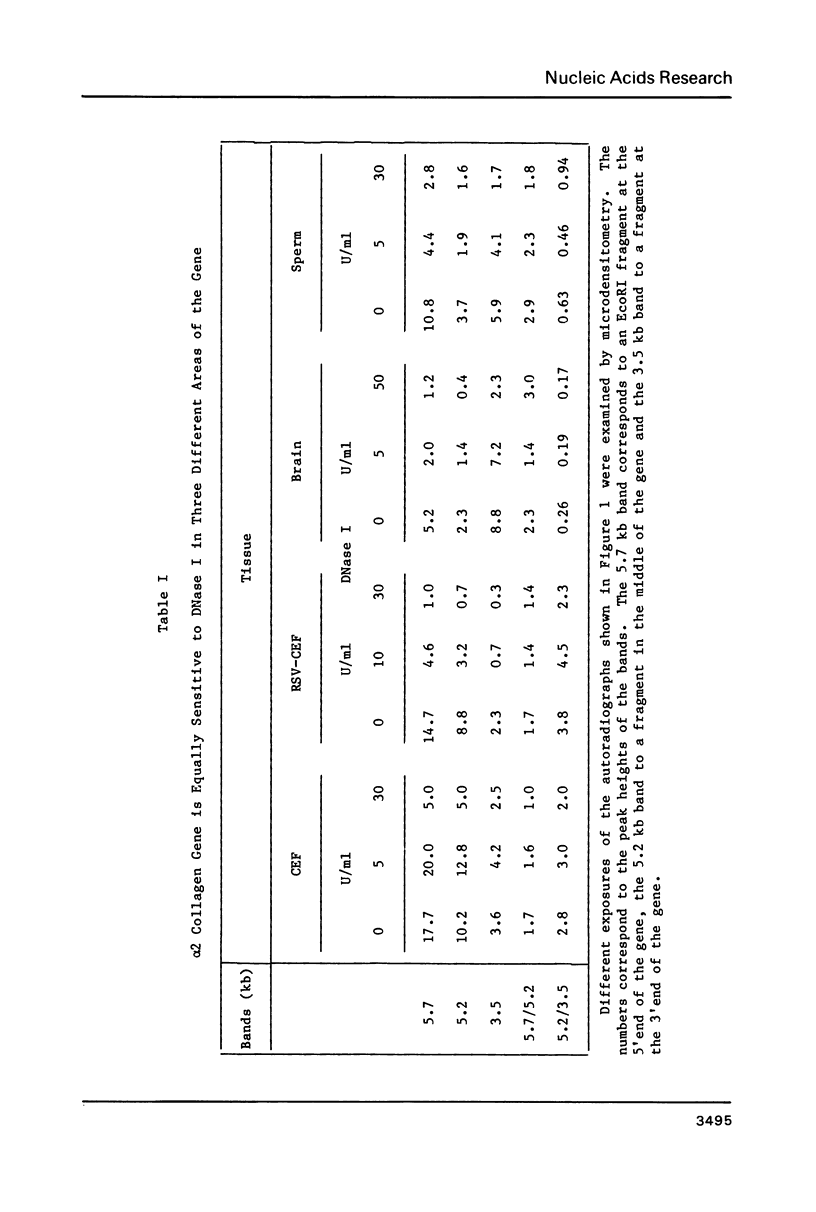
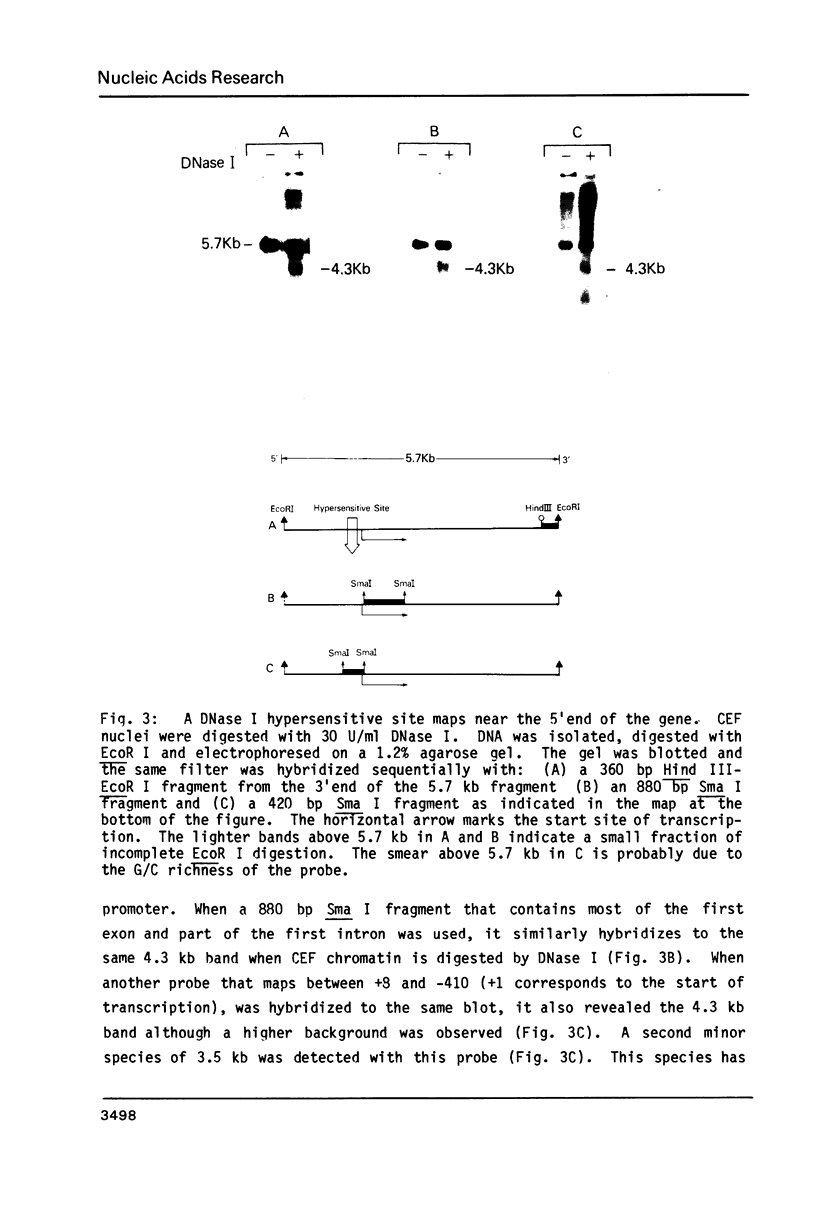
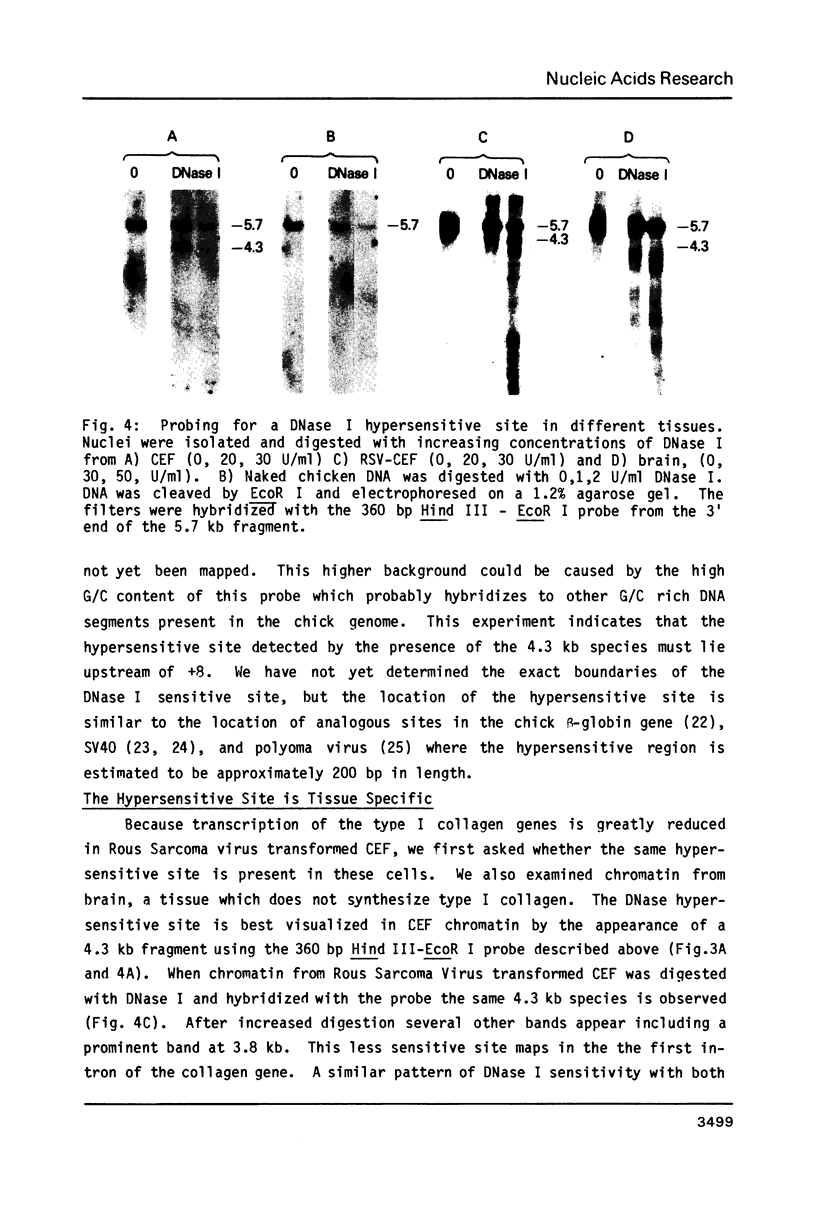
The chromatin structure of the chick alpha 2(I) collagen gene was probed with DNase I. Because our previous work strongly suggested that the 5' end of this gene is not methylated whereas the rest of the gene is methylated whether or not the gene is expressed, we compared the relative DNase I sensitivity of the methylated and unmethylated segments. Both regions demonstrate similar relative DNase I sensitivities within a given tissue. In chromatin of chick embryo fibroblasts, we find a DNase I hypersensitive site which maps between 100 and 300 bp preceding the start of transcription. This site is not found in brain chromatin but is present in chick embryo fibroblasts transformed by Rous Sarcoma virus although the rate of transcription of the alpha 2(I) collagen gene is greatly reduced in these cells. Hence, the mechanism responsible for the large decrease in alpha 2(I) collagen gene expression in RSV transformed cells is different from the mechanism that is responsible for the presence of a DNase I hypersensitive site in the promoter. Furthermore, changes in the DNase I sensitivity of the chromatin of the alpha 2(I) collagen promoter occur without changes in the methylation pattern of the gene.
Full text
PDF


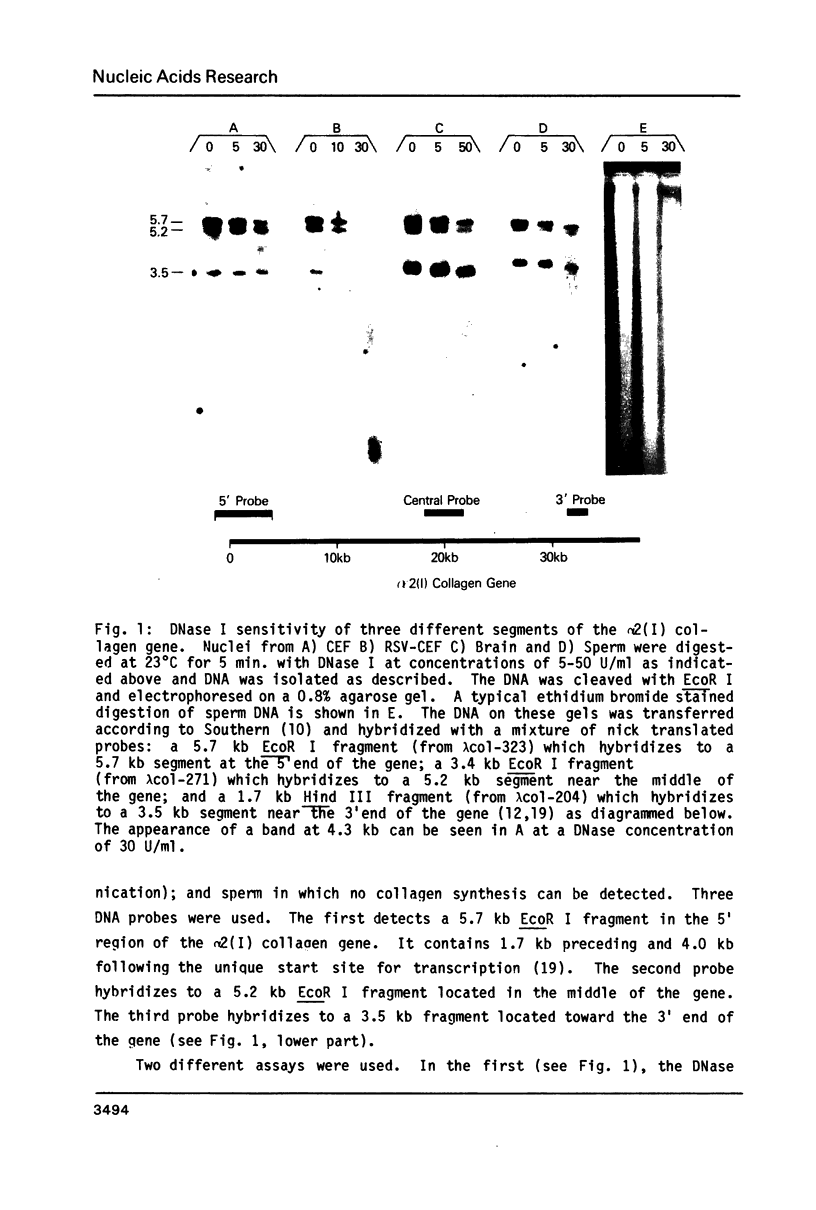





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Avvedimento E., Yamada Y., Lovelace E., Vogeli G., de Crombrugghe B., Pastan I. Decrease in the levels of nuclear RNA precursors for alpha 2 collagen in Rous sarcoma virus transformed fibroblasts. Nucleic Acids Res. 1981 Mar 11;9(5):1123–1131. doi: 10.1093/nar/9.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellard M., Kuo M. T., Dretzen G., Chambon P. Differential nuclease sensitivity of the ovalbumin and beta-globin chromatin regions in erythrocytes and oviduct cells of laying hen. Nucleic Acids Res. 1980 Jun 25;8(12):2737–2750. doi: 10.1093/nar/8.12.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Taggart M. H., Bird A. P. Unmethylated domains in vertebrate DNA. Nucleic Acids Res. 1983 Feb 11;11(3):647–658. doi: 10.1093/nar/11.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. DNAase I-hypersensitive sites of chromatin. Cell. 1981 Dec;27(3 Pt 2):413–415. doi: 10.1016/0092-8674(81)90381-0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Goldberg B., Todaro G. J. Differentiated cell types and the regulation of collagen synthesis. Nature. 1966 Nov 5;212(5062):631–633. doi: 10.1038/212631b0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of globin genes during chicken development. Cell. 1981 May;24(2):393–401. doi: 10.1016/0092-8674(81)90329-9. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Saragosti S., Blangy D., Yaniv M. Fine structure of the origin-proximal DNAase I-hypersensitive region in wild-type and EC mutant polyoma. Cell. 1981 Sep;25(3):651–658. doi: 10.1016/0092-8674(81)90172-0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Martinson H. G. Nucleosomes will not form on double-stranded RNa or over poly(dA).poly(dT) tracts in recombinant DNA. Nucleic Acids Res. 1981 Dec 21;9(24):6869–6888. doi: 10.1093/nar/9.24.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Macleod D., Bird A. DNAase I sensitivity and methylation of active versus inactive rRNA genes in xenopus species hybrids. Cell. 1982 May;29(1):211–218. doi: 10.1016/0092-8674(82)90105-2. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., McKeon C., de Crombrugghe B., Pastan I. Regulation of the expression of genes encoding types I, II, and III collagen during chick embryonic development. J Biol Chem. 1983 Aug 25;258(16):10041–10048. [PubMed] [Google Scholar]
- Nedospasov S. A., Georgiev G. P. Non-random cleavage of SV40 DNA in the compact minichromosome and free in solution by micrococcal nuclease. Biochem Biophys Res Commun. 1980 Jan 29;92(2):532–539. doi: 10.1016/0006-291x(80)90366-6. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Vogeli G., Mudryj M., Avvedimento V. E., Sullivan M., Pastan I., de Crombrugghe B. Isolation and characterization of overlapping genomic clones covering the chicken alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7059–7063. doi: 10.1073/pnas.77.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Sheffery M., Rifkind R. A., Marks P. A. Murine erythroleukemia cell differentiation: DNase I hypersensitivity and DNA methylation near the globin genes. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1180–1184. doi: 10.1073/pnas.79.4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Künzler P. Cromatin and core particles formed from the inner histones and synthetic polydeoxyribonucleotides of defined sequence. Nucleic Acids Res. 1979 Apr;6(4):1387–1415. doi: 10.1093/nar/6.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel M. R., Yamamoto T., de Crombrugghe B., Pastan I. Regulation or procollagen messenger ribonucleic acid levels in Rous sarcoma virus transformed chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2678–2684. doi: 10.1021/bi00512a049. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogeli G., Avvedimento E. V., Sullivan M., Maizel J. V., Jr, Lozano G., Adams S. L., Pastan I., de Crombrugghe B. Isolation and characterization of genomic DNA coding for alpha 2 type I collagen. Nucleic Acids Res. 1980 Apr 25;8(8):1823–1837. doi: 10.1093/nar/8.8.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli G., Ohkubo H., Sobel M. E., Yamada Y., Pastan I., de Crombrugghe B. Structure of the promoter for chicken alpha 2 type I collagen gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5334–5338. doi: 10.1073/pnas.78.9.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. A dominant role for DNA secondary structure in forming hypersensitive structures in chromatin. Cell. 1983 Apr;32(4):1191–1203. doi: 10.1016/0092-8674(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]