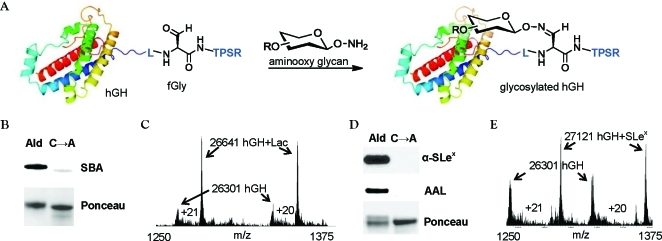
Figure 2.
Generation of site-specifically glycosylated hGH. (A) Aldehyde-tagged hGH was reacted with synthetic aminooxy glycans to yield the oxime-glycosylated conjugates. (B) Western blot of aldehyde-tagged hGH (lane 1) or the C→A mutant (lane 2) after reaction with aminooxy lactose S1 (5% MeCN, 0.02% FA, 0.26 mg/mL aldehyde-tagged hGH, 1 mM S1, 16 h, 37 °C). The blot was probed with SBA-FITC (top), and total protein was detected by Ponceau stain (bottom). (C) ESI-MS spectrum of the crude reaction mixture showing ions in the 20+ and 21+ charge states. Ions corresponding to aldehyde-tagged hGH (hGH) and glycosylated hGH (hGH+Lac) are shown with arrows. (D) Western blot of aldehyde-tagged hGH (lane 1) or the C→A mutant (lane 2) after reaction with aminooxy SLex38 (50 mM NaCit, pH 3.5, 0.26 mg/mL aldehyde-tagged hGH, 1 mM 38, 16 h, 37 °C). The blot was probed with an anti-SLex antibody KM93 followed by a horseradish peroxidase (HRP)-conjugated secondary antibody (top), and then stripped and reprobed with AAL-biotin followed by a FITC-conjugated antibiotin antibody (middle). Total protein loading was confirmed by Ponceau staining (bottom). (E) ESI-MS spectrum of the SLex crude reaction mixture showing ions in the 20+ and 21+ charge states. Ions corresponding to aldehyde-tagged hGH (hGH) and glycosylated hGH (hGH+SLex) are shown with arrows.