Abstract
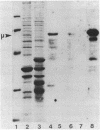
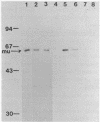
Genes for a murine mu heavy chain and a lambda light chain immunoglobulin have been inserted into bacterial expression plasmids containing the Escherichia coli trp promoter and ribosome binding site. Induction of transcription from the trp promoter results in accumulation of both light and heavy chain polypeptides in appropriate host strains. Both proteins were found as insoluble products. Following extraction and purification of the immunoglobulin containing fractions, antigen binding activity was recovered. The activity demonstrates essentially the same properties as the antibody from the hybridoma from which the genes were cloned.
Full text
PDF



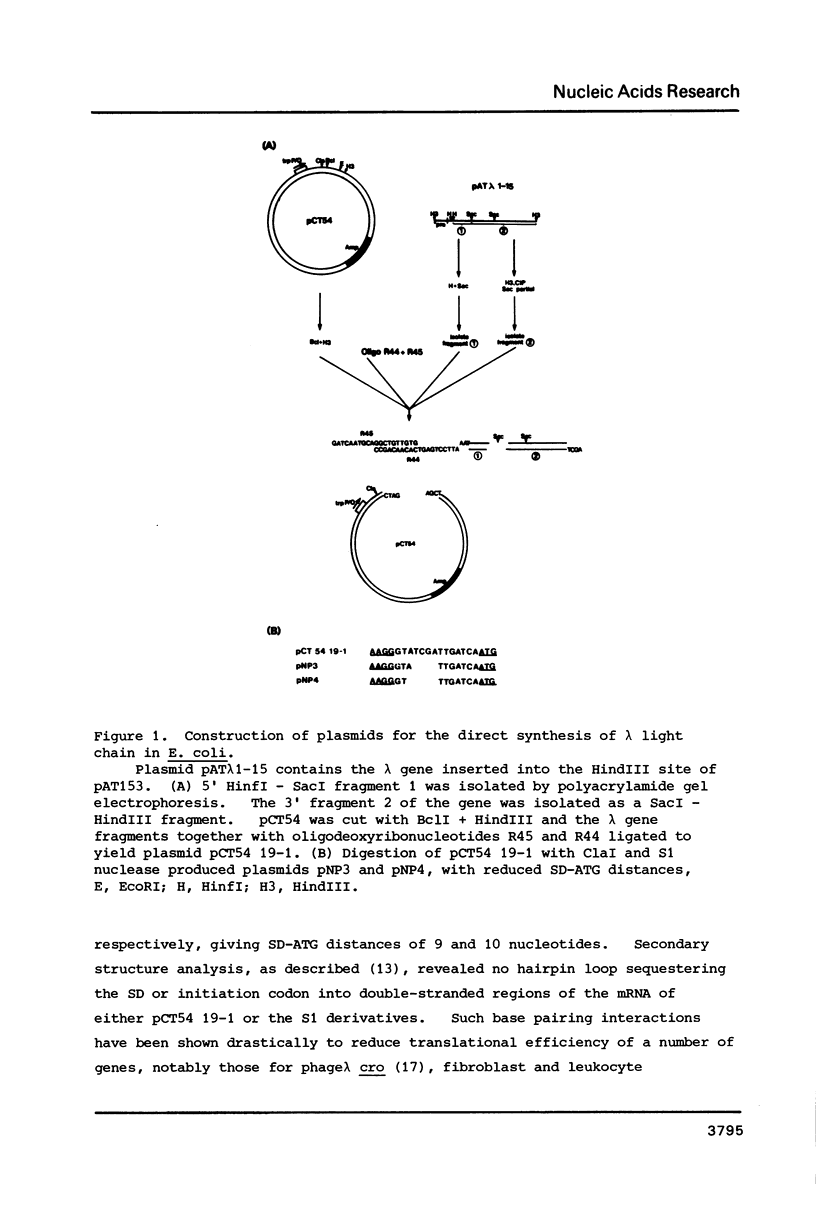
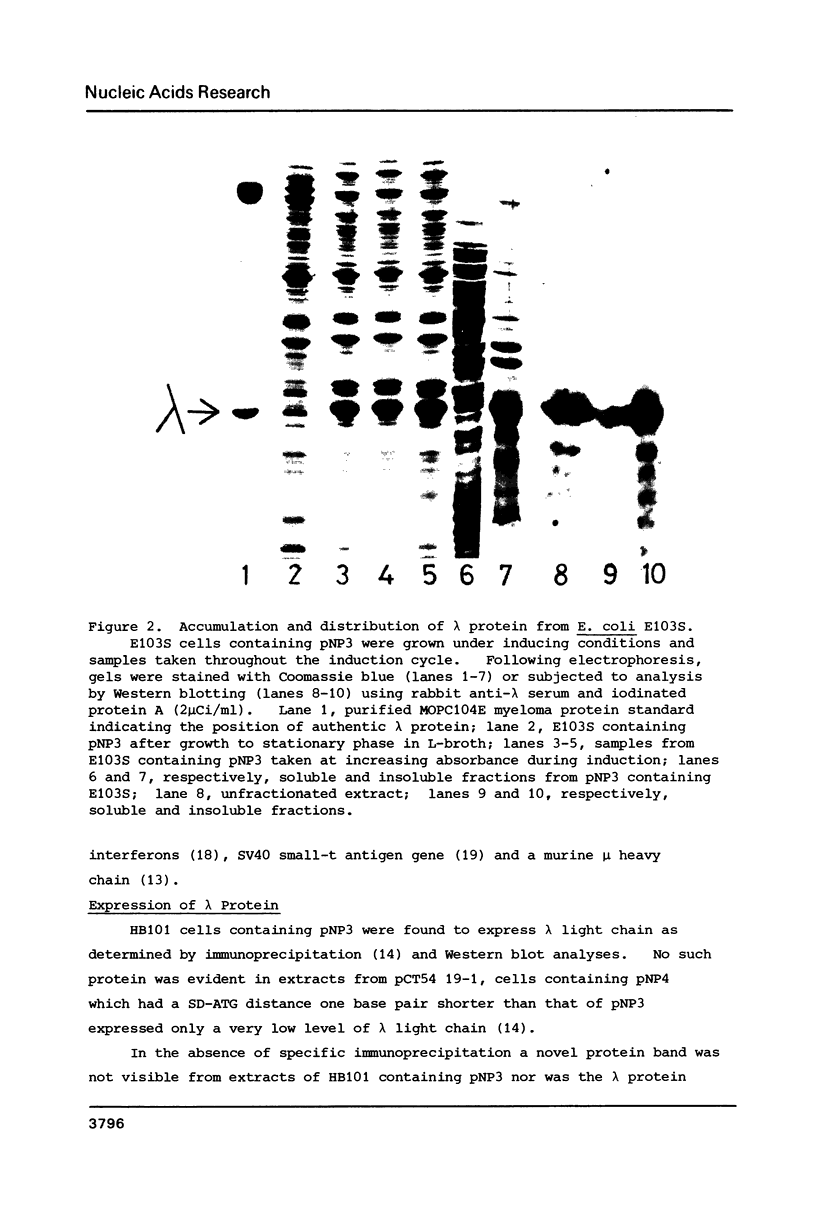




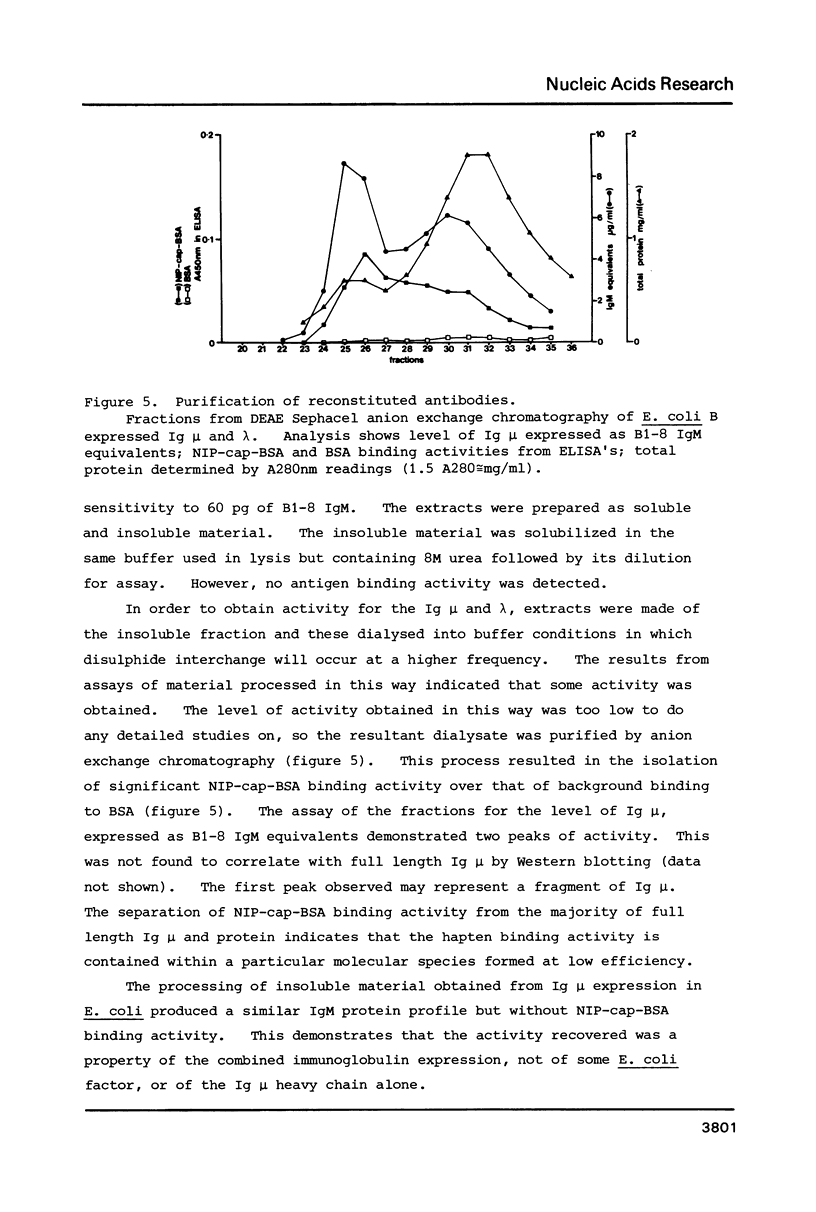
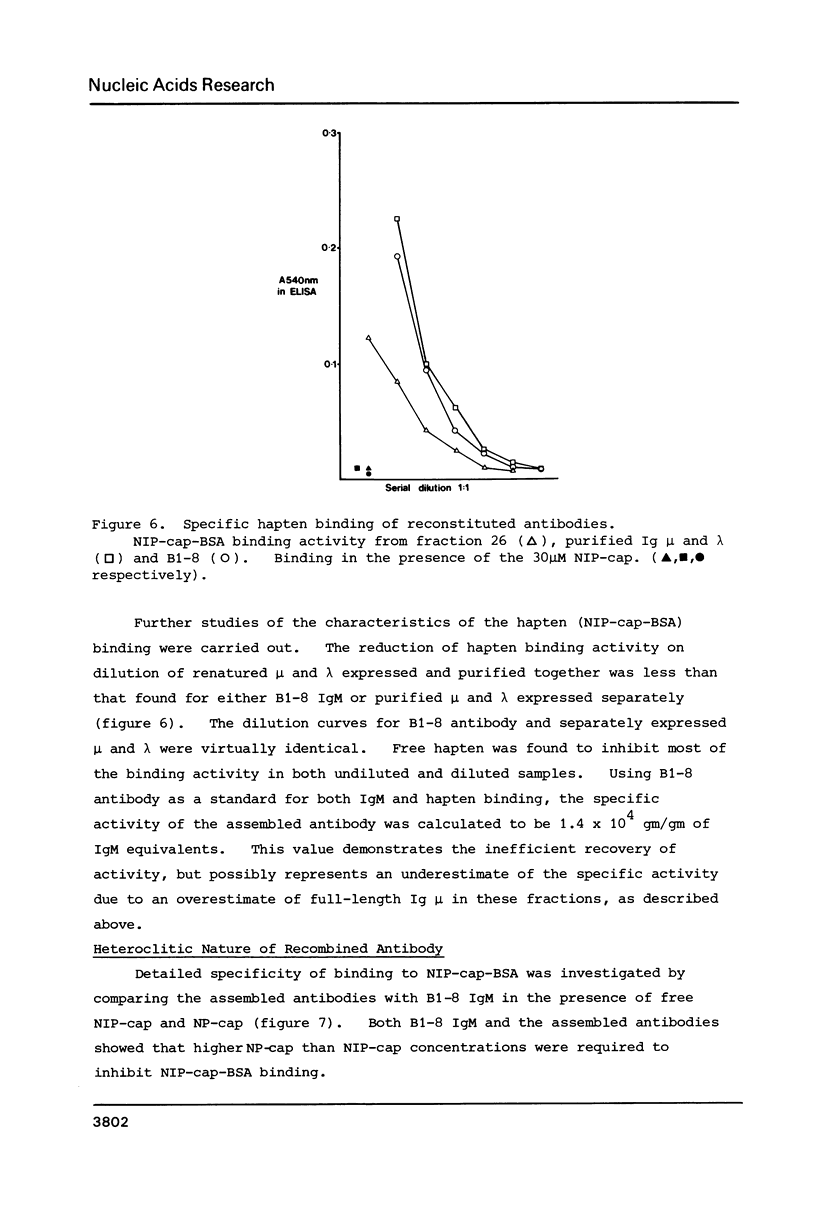
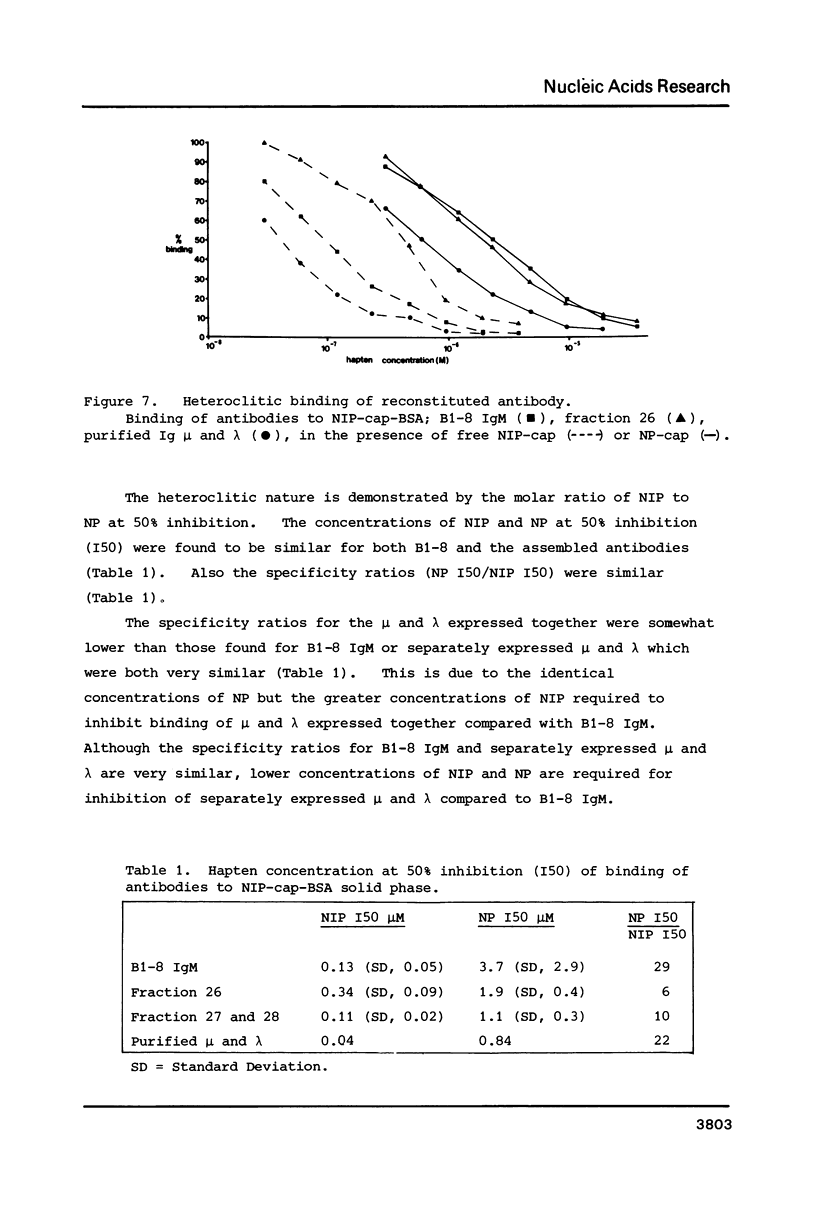



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amster O., Salomon D., Zemel O., Zamir A., Zeelon E. P., Kantor F., Schechter I. Synthesis of part of a mouse immunoglobulin light chain in a bacterial clone. Nucleic Acids Res. 1980 May 10;8(9):2055–2065. doi: 10.1093/nar/8.9.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Somatic variants of murine immunoglobulin lambda light chains. Nature. 1982 Jul 22;298(5872):380–382. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- Bridges S. H., Little J. R. Recovery of binding activity in reconstituted mouse myeloma proteins. Biochemistry. 1971 Jun 22;10(13):2525–2530. doi: 10.1021/bi00789a016. [DOI] [PubMed] [Google Scholar]
- Donch J., Chung Y. S., Greenberg J. Locus for radiation resistance in Escherichia coli strain B-r. Genetics. 1969 Feb;61(2):363–370. doi: 10.1093/genetics/61.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. The transcription termination site at the end of the early region of bacteriophage T7 DNA. Nucleic Acids Res. 1980 May 24;8(10):2119–2132. doi: 10.1093/nar/8.10.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage J. S., Angal S., Doel M. T., Harris T. J., Jenkins B., Lilley G., Lowe P. A. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3671–3675. doi: 10.1073/pnas.80.12.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D., Iserentant D., Derom C., Fiers W. Systematic alteration of the nucleotide sequence preceding the translation initiation codon and the effects on bacterial expression of the cloned SV40 small-t antigen gene. Gene. 1982 Jan;17(1):55–63. doi: 10.1016/0378-1119(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Imanishi T., Mäkelä O. Strain differences in the fine specificity of mouse anti-hapten antibodies. Eur J Immunol. 1973 Jun;3(6):323–330. doi: 10.1002/eji.1830030602. [DOI] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa T., Seno M., Sasada R., Ono Y., Onda H., Igarashi K., Kikuchi M., Sugino Y., Honjo T. Expression of human immunoglobulin E epsilon chain cDNA in E. coli. Nucleic Acids Res. 1983 May 25;11(10):3077–3085. doi: 10.1093/nar/11.10.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. P., Millican T. A., Bose C. C., Titmas R. C., Mock G. A., Eaton M. A. Improvements to solid phase phosphotriester synthesis of deoxyoligonucleotides. Nucleic Acids Res. 1982 Sep 25;10(18):5605–5620. doi: 10.1093/nar/10.18.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Shepard H. M., Yelverton E., Goeddel D. V. Increased synthesis in E. coli of fibroblast and leukocyte interferons through alterations in ribosome binding sites. DNA. 1982;1(2):125–131. doi: 10.1089/dna.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
- Yelverton E., Norton S., Obijeski J. F., Goeddel D. V. Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science. 1983 Feb 11;219(4585):614–620. doi: 10.1126/science.6297004. [DOI] [PubMed] [Google Scholar]