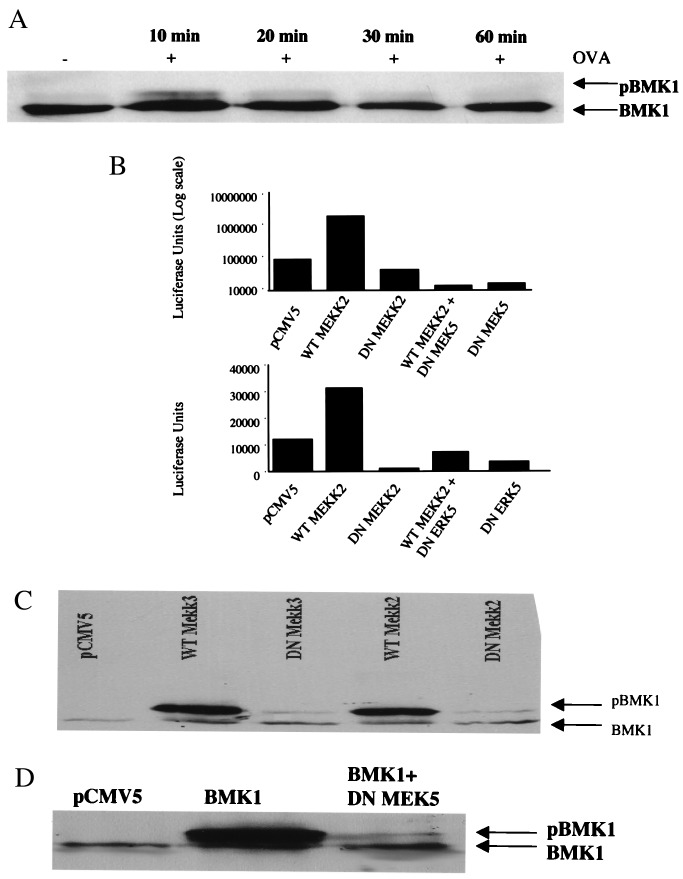
Figure 7.
Expression of kinase-inactive MEK5 inhibits phosphorylation of BMK1 and the promoter activity of TNF-α. (A) MC/9 cells (10 × 106 cells) were stimulated with 10 μg/ml OVA (IgE sensitized) for a time course of 0, 10, 20, 30, or 60 min. BMK1 phosphorylation was analyzed by the mobility shift of proteins on a Western blot and visualization by chemiluminescence. (B) MC/9 cells (10 × 106 cells) were transfected with 5 μg pGL3TNF together with 7.5 μg MEKK2 or 7.5 μg of MEKK2 K/R; or 7.5 μg MEK5 S311A, S315A, or dominant negative ERK 5; or an equivalent amount of empty vector (pCMV5). The transfected cells were passively sensitized with anti-OVA IgE for 16 h and washed three times and incubated for an additional 6 h with 10 μg/ml OVA or PBS. Luciferase activities are shown as relative light units (luciferase units) (representative of three separate experiments). (C and D) MC/9 cells were transfected with 15 μg of WT MEKK2 and WT MEKK3 or 15 μg kinase-inactive MEKK2 (K-M) and kinase-inactive MEKK3 (K-M) or DN MEK5 (D), along with 5 μg of BMK1α plasmid or an equivalent amount of empty vector (pCMV5). Phosphorylation of BMK1 was analyzed by a shift in its electrophoretic mobility detected by Western blotting.