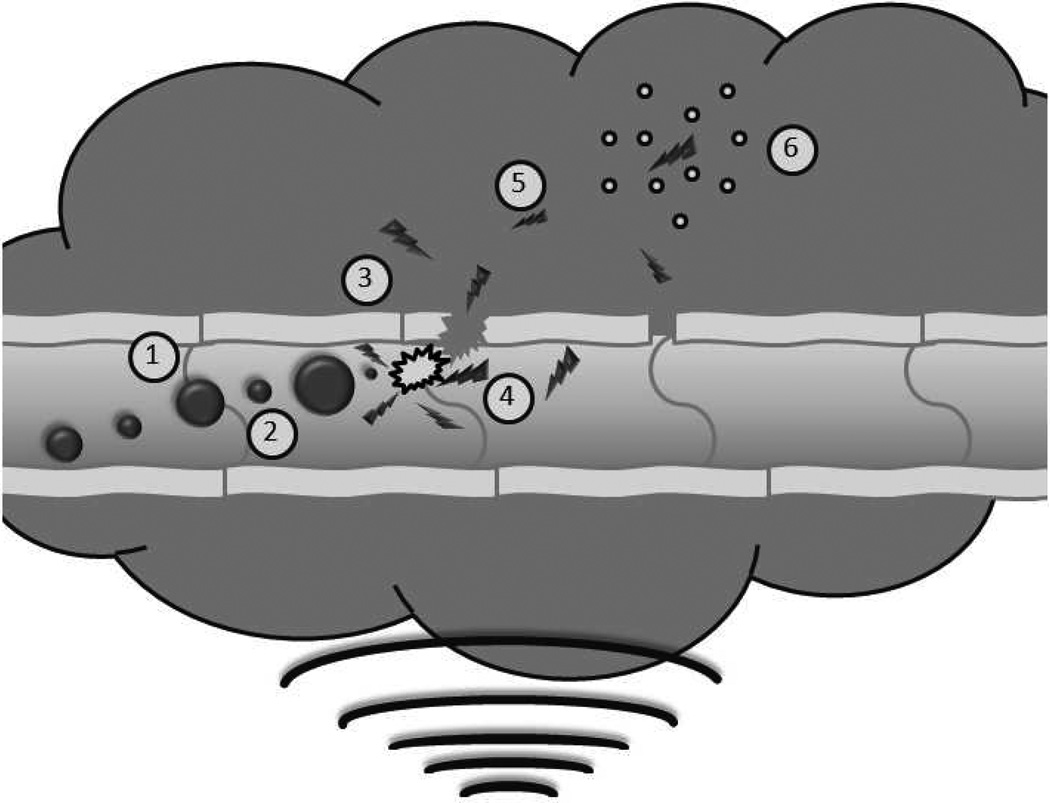
Figure 1.
Schematic of ultrasound triggered drug delivery. Doxorubicin loaded UCAs pass freely through the vasculature until exposed to ultrasound where they experience (1) acoustic radiation forces that push the microbubbles to the vessel wall. The oscillating pressure wave will also lead to (2) microbubble cavitation as the gas core expands and contracts in response to changes in pressure. When exposed to sufficiently strong ultrasound pulses, the microbubble will undergo (3) inertial cavitation resulting in the destruction of the polymer shell, creating drug loaded polymer fragments less than 400 nm. The energy released in the process of microbubble destruction is sufficient to (4) enhance the permeability of the vessel wall. The fragments can then begin to (5) accumulate within the tumor interstitium, potentially, through acoustic radiation force [39] and with time, circulating fragments will accumulate though the enhanced permeability and retention effect. Lodged polymer fragments can (6) slowly degrade providing a sustained localized release of the chemotherapeutic agent.