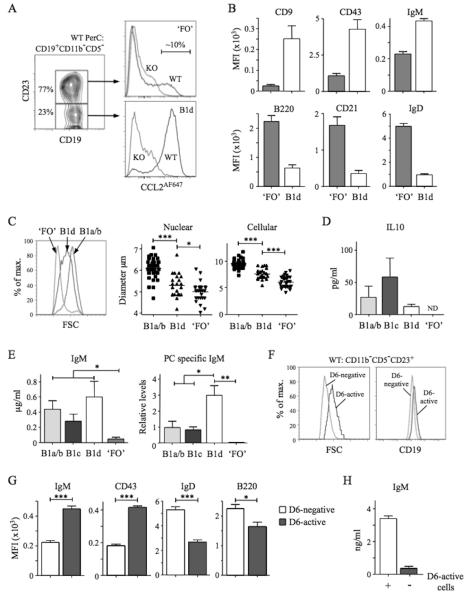
Figure 4. Identification and characterisation of PerC B1d and B1d23+ cells.
(A) Left panel: Sub-fractionation of WT PerC CD19+CD11b−CD5− cells by anti-CD23. The abundance of the two subsets is shown as the mean percentage of total CD19+CD11b−CD5− cells. Right panels: Overlaid CCL2AF647 uptake profiles of WT (black) and D6-deficient (KO, grey) CD23+ and CD23− subsets, referred to as ‘FO’ and B1d, respectively. Proportion of D6active ‘FO’ B cells is indicated. (B) Surface immunophenotype of WT PerC ‘FO’ and B1d B cells. Data show average Mean Fluorescence Intensity (+/−SEM). (C) Comparison of size of WT PerC ‘FO’, B1d and CD11b+ B1 (B1a/b) cells by forward scatter (FSC) (left panel), and microscopic measurements of nuclear and cellular diameter (middle and right panels) of FACS-sorted PerC cells. (D-E) Production of IL-10, IgM, and anti-PC Ab by FACS-sorted, cultured CD11b+ B1 (B1a/b), B1c, B1d, and ‘FO’ cells. Data show mean (+/−SEM) of data generated from 3 repeat experiments. ND, not detected. (F) Overlaid forward scatter (FSC) (left panel) and surface CD19 (right panel) of WT PerC D6- active and D6-negative CD19+CD11b−CD5−CD23+ ‘FO’ cells. (G) Surface immunophenotype of WT PerC D6-active and D6-negative CD19+CD11b−CD5−CD23+ ‘FO’ cells (average Mean Fluorescence Intensity (+/−SEM)). (H) Production of IgM by FACS-sorted, cultured CD19+CD11b−CD5−CD23+ cells in the absence (−) or presence (+) of their D6-active subset. Flow cytometry profiles are representative of data from at least 3 mice per genotype per experiment, with experiments repeated on at least 3 occasions. *p<0.05; **p<0.01; ***p<0.001.