Fig. 7.
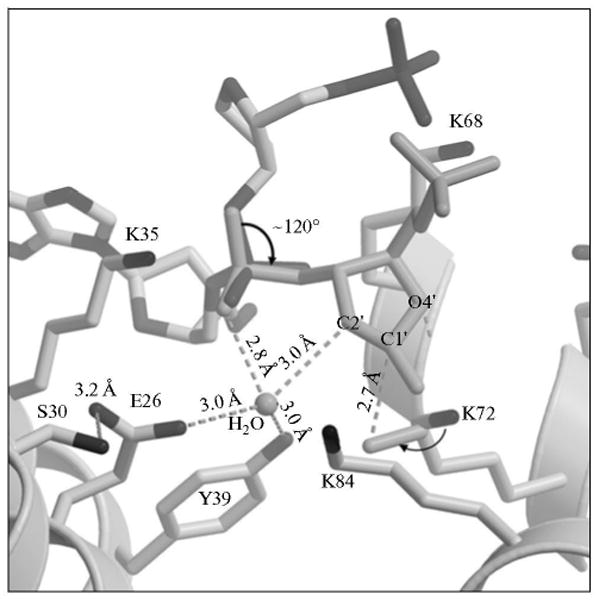
A model of a proposed conformational change in the dRP group. Key amino acid residues of the polymerase β lyase active site are indicated. Rotation of the sugar phosphate (thick arrow), along with additional small adjustments, adequately positions reactive groups necessary for catalysis. Dashed lines indicate distances between the catalytic residues and reactive groups. The polymerase β side chains and the sugar ring atoms are labeled. A water molecule in the active site is labeled. The attack of the K72 side chain nitrogen on the C1′ over 2.7 Å is illustrated.
