Fig. 2. Affinity labeling of membrane bound binding site with the 4-azidobenzoimidate derivative of 125I-α-bungarotoxin in the presence of increasing amounts of acetylcholine (A) and α-bungarotoxin (B).
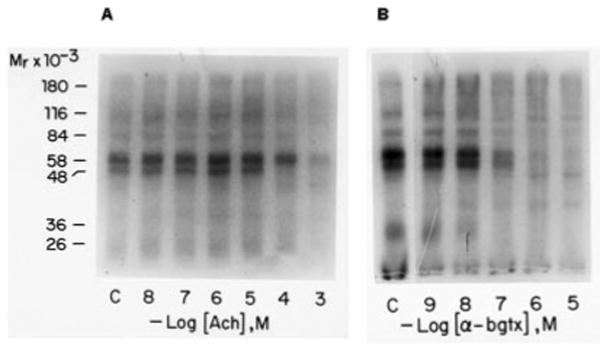
A, membranes prepared from Drosophila heads were preincubated with increasing amounts of acetylcholine for 20 min in the presence of 1 μm of the cholinesterase inhibitor physostigmine. The presence of the inhibitor had no effect on the binding of 125I-α-bungarotoxin or its photoactivatable derivative (data not shown). B, membranes prepared from Drosophila heads were preincubated with increasing amounts of α-bungarotoxin (α-bgtx). Photoaffinity labeling was carried out in both experiments as described under experimental procedures. Following labeling membranes were collected by centrifugation and analyzed by SDS-polyacrylamide gel electrophoresis. An autoradiogram of the dried gel is shown. Cross-linking with α-bungarotoxin increases the molecular mass of each band by about 8 kDa.
