Fig. 4. SDS-polyacrylamide gel electrophoresis of the main fractions obtained during the receptor protein purification.
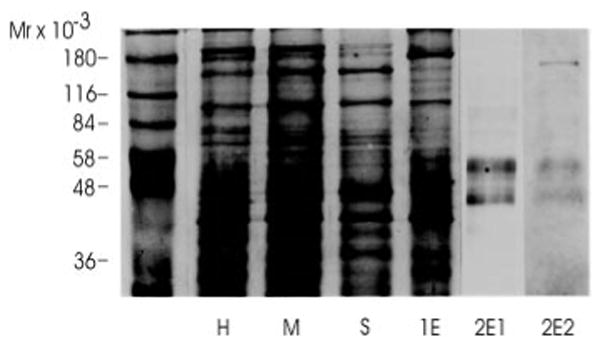
Aliquots of the main fractions obtained during the purification were analyzed by SDS-polyacrylamide gel electrophorsis on a 10% polyacrylamide gel. The equivalent of 10 μg of protein was loaded in lanes H, M, S, and 1E. 1.5 μg of protein was loaded in lanes 2E1 and 2E2. Following electrophoresis proteins were visualized by silver staining with the exception of lane 2E2, which was stained with Coomassie Blue. H, head homogenate; M, membrane fraction; S, solubilized binding protein fraction; 1E, eluate from the cobratoxin affinity column; 2E1 and 2E2, eluates of the lentil lectin affinity column after concentration.
