Fig. 5. Analysis of the purified binding protein fraction.
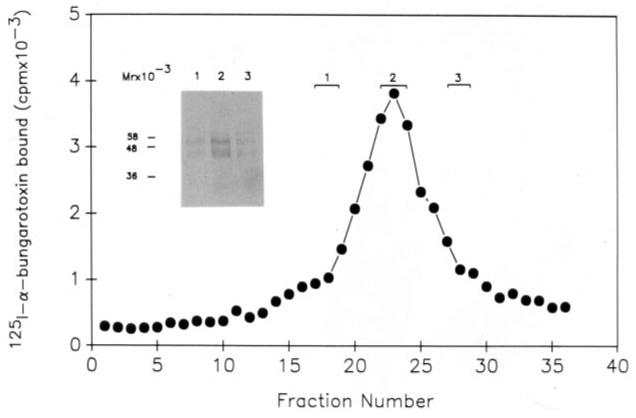
26 μg of purified binding protein fraction was layered on a sterile 5–20% sucrose gradient as described under “Materials and Methods.” Centrifugation was carried out in a Beckman SW 50.1 rotor at 45,000 rpm for 12 h at 4 °C. Fractions of 110 μl were collected from the gradient and analyzed for α-bungarotoxin binding activity. Fractions obtained from the gradient were pooled as indicated in the figure and the proteins in the three pools precipitated quantitatively as described under “Materials and Methods.” The precipitated proteins from the three pools were analyzed by SDS-polyacrylamide gel electrophoresis corresponding to lanes 1, 2, and 3 on the inserted panel and visualized by silver staining.
