Abstract
In 4M NaCl solutions (dC-dG)n (n = 3,4; approximately 9 mM) exist as a mixture o +/- B and Z forms. The low and high field components of two 31P NMR resonances originating from internal phosphodiester groups are assigned to the GpC and CpG linkages, respectively. Low temperatures stabilize the Z-forms, which completely disappear above 50 degrees C (n = 3) and 65 degrees C (n = 4). delta H = -44 and -17 kJ/mol for B to Z transition in the hexamer and octamer duplexes, respectively. Temperature dependent changes (0-50 degrees C range) in the spin-lattice relaxation times at 145.7 MHz are distinctly different for the 31P nuclei o +/- GpC and CpG groups. The relaxation data can be explained by assuming that the GpC phosphodiester groups undergo more local internal motion than do the CpG groups.
Full text
PDF

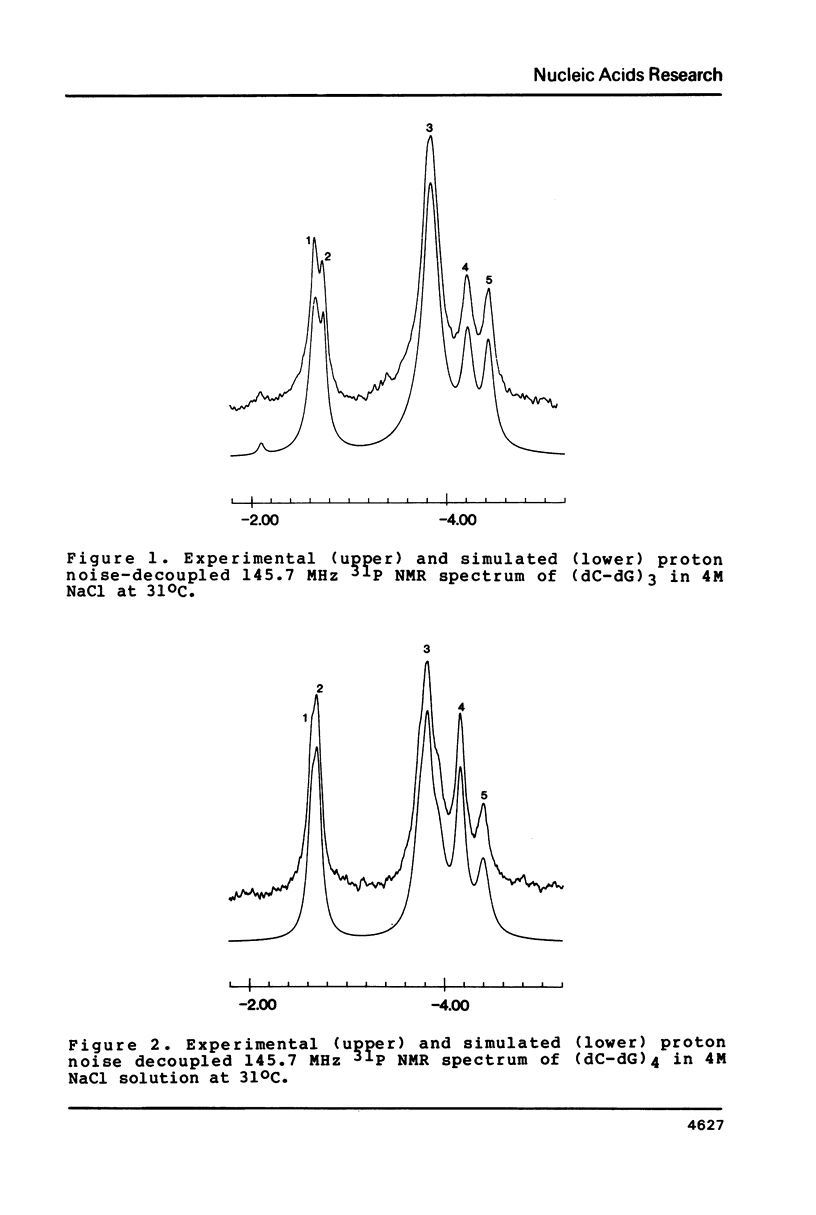


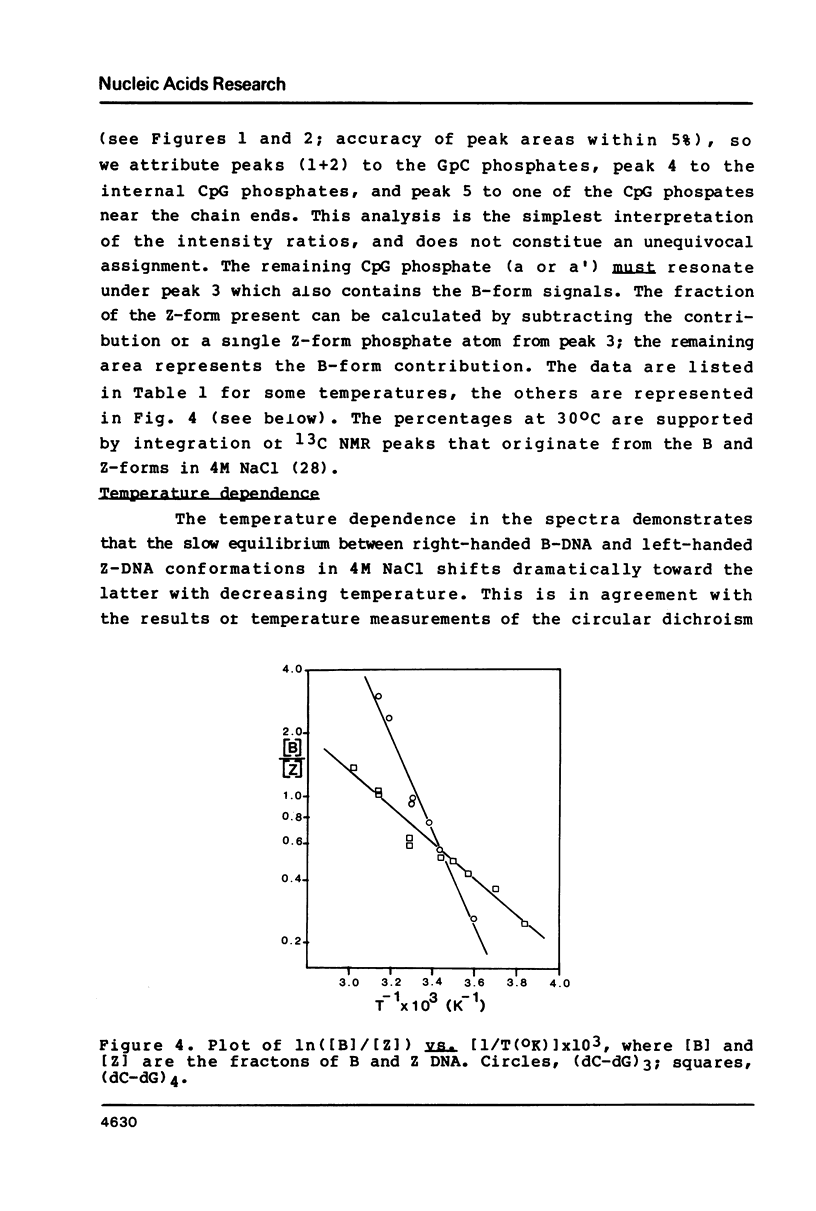





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison S. A., Shibata J. H., Wilcoxon J., Schurr J. M. NMR relaxation in DNA. I. The contribution of torsional deformation modes of the elastic filament. Biopolymers. 1982 Apr;21(4):729–762. doi: 10.1002/bip.360210403. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291x(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Cohen J. S. Salt- and sequence-dependence of the secondary structure of DNA in solution by 31P-NMR spectroscopy. Biopolymers. 1983 Mar;22(3):879–893. doi: 10.1002/bip.360220310. [DOI] [PubMed] [Google Scholar]
- Chen C., Cohen J. S., Behe M. B to Z transition of double-stranded poly[deoxyguanylyl(3'-5')-5-methyldeoxycytidine] in solution by phosphorus-31 and carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Apr 26;22(9):2136–2142. doi: 10.1021/bi00278a013. [DOI] [PubMed] [Google Scholar]
- Cheng D. M., Kan S. L., Miller P. S., Leutzinger E. E., Ts'o P. O. An effective method for the assignment of 31P-NMR resonance(s) of oligonucleotides. Biopolymers. 1982 Mar;21(3):697–701. doi: 10.1002/bip.360210315. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Jovin T. M. Assignment of resonances in the phosphorus-31 nuclear magnetic resonance spectrum of poly[d(A-T)] from phosphorothioate substitution. Biochemistry. 1983 Sep 13;22(19):4546–4550. doi: 10.1021/bi00288a030. [DOI] [PubMed] [Google Scholar]
- Feigon J., Wang A. H., van der Marel G. A., Van Boom J. H., Rich A. A one- and two-dimensional NMR study of the B to Z transition of (m5dC-dG)3 in methanolic solution. Nucleic Acids Res. 1984 Jan 25;12(2):1243–1263. doi: 10.1093/nar/12.2.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein D. G., Luxon B. A., Goldfield E. M., Lai K., Vegeais D. Phosphorus-31 nuclear magnetic resonance of double- and triple-helical nucleic acids. Phosphorus-31 chemical shifts as a probe of phosphorus-oxygen ester bond torsional angles. Biochemistry. 1982 Feb 2;21(3):580–589. doi: 10.1021/bi00532a026. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G. Nucleotide conformational analysis by 31P nuclear magnetic resonance spectroscopy. Annu Rev Biophys Bioeng. 1981;10:355–386. doi: 10.1146/annurev.bb.10.060181.002035. [DOI] [PubMed] [Google Scholar]
- Hartmann B., Thuong N. T., Pouyet J., Ptak M., Leng M. Spectroscopic studies of (m5dC-dG)3: thermal stability of B- and Z-forms. Nucleic Acids Res. 1983 Jul 11;11(13):4453–4466. doi: 10.1093/nar/11.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F., Akasaka K., Hatano H. 31P Magnetic relaxation studies of yeast transfer RNAPhe. Biopolymers. 1977 Mar;16(3):655–667. doi: 10.1002/bip.1977.360160314. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Kypr J., Vorlícková M., Budesinský M., Sklenár V. Strange double helix of poly (dA-dT) in high-salt solution. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1257–1264. doi: 10.1016/0006-291x(81)90755-5. [DOI] [PubMed] [Google Scholar]
- Levy G. C., Craik D. J., Kumar A., London R. E. A critical evaluation of models for complex molecular dynamics: application of NMR studies of double- and single-stranded DNA. Biopolymers. 1983 Dec;22(12):2703–2726. doi: 10.1002/bip.360221214. [DOI] [PubMed] [Google Scholar]
- Levy G. C., Marchetti P. S., Ejchart A., Levy L. F., Kumar A., Hilliard P. R., Rill R. L. Understanding DNA conformational dynamics: answering questions and questioning answers. J Biomol Struct Dyn. 1983 Dec;1(3):795–808. doi: 10.1080/07391102.1983.10507482. [DOI] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Morris M. E., Levy G. Absorption of sulfate from orally administered magnesium sulfate in man. J Toxicol Clin Toxicol. 1983 Apr;20(2):107–114. doi: 10.3109/15563658308990056. [DOI] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Kozlowski S. A., Patel D. J., Rich A. Bromination stabilizes poly(dG-dC) in the Z-DNA form under low-salt conditions. Biochemistry. 1984 Jan 3;23(1):54–62. doi: 10.1021/bi00296a009. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Helix-coil transition of the self-complementary dG-dG-dA-dA-dT-dT-dC-dC duplex. Eur J Biochem. 1979 May 15;96(2):267–276. doi: 10.1111/j.1432-1033.1979.tb13037.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrifoglio F., Manzini G., Vasser M., Dinkelspiel K., Crea R. Conformational stability of alternating d (CG) oligomers in high salt solution. Nucleic Acids Res. 1981 May 11;9(9):2195–2206. doi: 10.1093/nar/9.9.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rill R. L., Hilliard P. R., Jr, Levy G. C. Spontaneous ordering of DNA. Effects of intermolecular interactions on DNA motional dynamics monitored by 13C and 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1983 Jan 10;258(1):250–256. [PubMed] [Google Scholar]
- Shindo H., Simpson R. T., Cohen J. S. An alternating conformation characterizes the phosphodiester backbone of poly(dA-dT) in solution. J Biol Chem. 1979 Sep 10;254(17):8125–8128. [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of 145 base pair length poly (dG-dC) . poly (dG-dC) in solution and in association with histones. Nucleic Acids Res. 1980 May 10;8(9):2093–2103. doi: 10.1093/nar/8.9.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]


