Abstract
BACKGROUND
Small animal models have been used in traumatic brain injury (TBI) research to investigate the basic mechanisms and pathology of TBI. Unfortunately, successful TBI investigations in small animal models have not resulted in marked improvements in clinical outcomes of TBI patients.
OBJECTIVE
To develop a clinically relevant immature large animal model of pediatric neurocritical care following TBI.
METHODS
Eleven 4 week old piglets were randomized to either rapid axial head rotation without impact (N=6) or instrumented sham (N=5). All animals had an intracranial pressure monitor, brain tissue oxygen (PbtO2) probe, and cerebral microdialysis probe placed in the frontal lobe and data collected for 6 h following injury.
RESULTS
Injured animals had sustained elevations in intracranial pressure and lactate-pyruvate ratio (LPR), and decreased PbtO2 compared to sham. PbtO2 and LPR from separate frontal lobes had strong linear correlation in both sham and injured animals. Neuropathologic examination demonstrated significant axonal injury and infarct volumes in injured animals compared to sham at 6 hours post-injury. Averaged over time, PbtO2 in both injured and sham animals had a strong inverse correlation with total injury volume. Average LPR had a strong correlation with total injury volume.
CONCLUSION
LPR and PbtO2 can be utilized as serial non-terminal secondary markers in our injury model for neuropathology, and as evaluation metrics for novel interventions and therapeutics in the acute post-injury period. This translational model bridges a vital gap in knowledge between TBI studies in small animal models and clinical trials in the pediatric TBI population.
Keywords: neurocritical care monitoring, pediatric head injury, swine, TBI model
INTRODUCTION
Traumatic brain injury (TBI) is the most common cause of death in childhood 1. Despite advances in resuscitation care, morbidity following pediatric TBI remains high. One of the principal goals of acute intensive care management of pediatric patients with TBI is the stabilization of derangements in cerebrovascular hemodynamics and oxygenation to avoid subsequent and additional neurotrauma 2. Therapies and interventions available to the pediatric intensivist or neurosurgeon to treat TBI are limited, and evidence for their efficacy is primarily based on adult or anecdotal data 2. Clinical trials in pediatric TBI are difficult to conduct due to the heterogeneous patient population and need for large multi-center studies 3.
Animal models have been used in traumatic brain injury research to investigate the basic mechanisms and pathology of TBI. These models offer the opportunity to simulate the sequence of events that occur in human TBI and investigate in detail the associated pathophysiology. Small animal (rodent) models have been used more frequently than large animal models to study TBI. Unfortunately, successful TBI investigations in small animal models have not resulted in marked improvements in clinical outcomes of TBI patients. Porcine models of TBI have some distinct advantages over rodent models. Rodents have little white matter and their brains are lissenencephalic, whereas pigs and humans have gyri and similar distribution of grey and white matter which may be critical for modeling some injury types such as diffuse axonal injury 4, 5.
Other advantages of porcine models include changes in composition of the brain during development that parallel human development, and a larger physical size of the brain permits clinically relevant intracranial monitoring (e.g. intracranial pressure, microdialysis, brain tissue oxygen content, and cerebral blood flow) 6-8. These characteristics make an immature pig model an attractive platform to test possible therapeutics or interventions in the acute care setting for improving pediatric TBI outcomes.
Several investigators have developed clinically relevant adult porcine models of TBI with clinically relevant intracranial monitoring. Alessandri et al developed a focal lesion model in mature swine with multimodal intracranial monitoring, including intracranial pressure (ICP) monitoring, brain tissue oxygenation, and microdialysis 9. This multimodal intracranial modeling was then applied to a porcine model of acute subdural hematoma 10. Manley et al also developed a focal TBI model in mature swine with ICP monitoring and investigated the effects of various impactor depths of depression on ICP and neuropathology 11. A limitation of these models is the need for opening the skull to apply the controlled cortical impact and the possibility of dura disruption resulting in lower ICP values. We previously reported our experience with an immature swine closed head injury model of diffuse white matter injury and hemorrhage and our development of neurobehavioral functional outcome measures that correlate with neuropathology 12-15. The purpose of this study was to develop a novel critical care model of pediatric closed head injury that utilizes a large animal model to enable full clinical modalities used in the neuro-intensive care setting, and to describe acute physiologic monitoring and pathology in this closed head injury model.
MATERIAL AND METHODS
Animal Preparation
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Eleven 4 week old piglets whose brain development, myelination, and cerebrovascular responses correspond to the human toddler were studied 16. Piglets were anesthetized with an intramuscular injection of ketamine (20mg/kg) and xlyazine (2 mg/kg) followed by inhaled 4% isoflurane. Vitals signs including heart rate, blood pressure, respiratory rate, end tidal CO2, pulse oximetry, and rectal temperature were monitored continuously and recorded every 15 minutes. Rectal temperature was maintained between 36 - 38 °C using a heating pad and lamp.
When a pinch reflex was absent, piglets were orally intubated with 4.5 mm endotracheal tubes and mechanically ventilated. Femoral artery and venous catheters were placed for continuous mean arterial blood pressure (MAP) monitoring, arterial blood gas sampling, and intravenous (IV) fluid administration. Animals were administered 50 mcg/kg fentanyl IV, followed by a fentanyl infusion at 50 mcg/kg/hr and normal saline at 4 mL/kg/hr. Following initiation of the fentanyl infusion, isoflurane was reduced to 0.5-1%. Mechanical ventilation was adjusted to maintain end tidal CO2 between 35-40 mm Hg and fraction of inspired oxygen was maintained between 28-30%. Pre- and post-injury arterial blood gas samples were obtained hourly and analyzed hourly pre and post injury (Nova Biomedical, Waltham, MA) until euthanasia 6 hours after injury.
Non-impact rotational brain injury
Animals were randomized to injured (INJ, N = 6), or sham (SHAM, N = 5). Injured animals experienced rapid axial head rotations without impact (angle rotation 90° over 10-12 msec) as described previously 12, 14, 15. Immediately prior to injury, isoflurane was discontinued. Angular velocity was measured using an angular rate sensor (ATA, Albuquerque, NM) attached to the linkage sidearm. Injured animals received an average peak angular velocity of 169.4 ± 2.9 rad/s. After injury, mechanical ventilation was continued and latency to return of pinch reflex following injury or sham was recorded and isoflurane 0.5-1% was resumed. The animal was removed from the bite-plate and placed in the prone position.
Neuromonitoring
After injury, three burr holes were prepared for placement of fiber-optic intracranial pressure monitor, brain tissue oxygen monitor, and microdialysis probe. (Figure 1). The fiber-optic intracranial pressure probe (Integra, Plainsboro, NJ) was secured with a single lumen bolt. The Licox catheter system (Integra, Plainsboro, NJ) was placed to measure brain tissue oxygenation (PbtO2). The brain tissue oxygen probe was inserted to a depth of 1.5 cm, secured with bone wax, and allowed to equilibrate for 30 minutes before values were recorded. A hyperoxia test was performed to confirm proper catheter placement. Data for intracranial pressure and PbtO2 were recorded every 15 minutes until sacrifice.
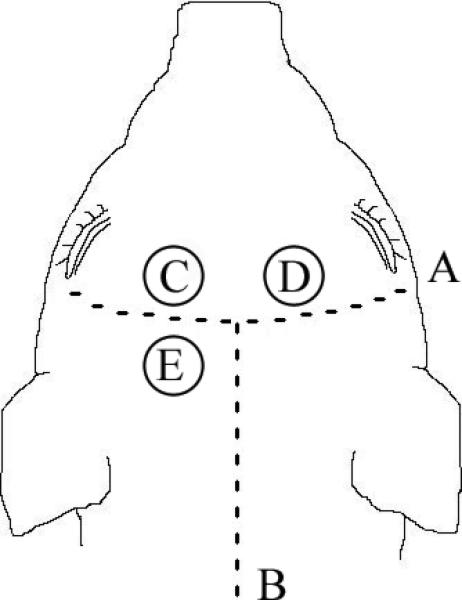
Figure 1.
Schematic of pig head illustrating placement of intracranial monitoring. (A) coronal suture, (B) sagittal suture, (C) brain tissue oxygen probe, (D) microdialysis probe, (E) intracranial pressure monitor
A microdialysis probe (PAS 12, 4mm length) was placed on the opposite side of the skull from the PbtO2 catheter (Figure 1). Immediately after insertion, the probe was infused with sterile 0.9% NaCl at a rate of 1 μL/min. Dialysate samples were collected every 30 minutes until sacrifice and stored at -80 C and analyzed for lactate and pyruvate using a CMA600 analyzer (CMA, North Chelmsford, MA).
Histology
At 6 hours post-injury, animals were sacrificed via an overdose of pentobarbital. Brains were perfusion-fixed using 0.9% NaCl followed by 10% neutral buffered formalin, removed, and post-fixed for more than 24 hours in 10% formalin. All gross and histopathological examinations were performed by a neuropathologist blinded to group assignment. Brains were sectioned into 3mm thick coronal blocks through the cerebrum, brain stem, and high cervical spinal cord, sections were grossly examined for tissue tears, intracerebral hemorrhage, and subarachnoid hemorrhage. Following routine processing, tissue was embedded in paraffin wax, and two 6 μm thick sections were cut from every block for microscopic evaluation. Sections were stained with Hematoxylin and Eosin (H&E), or with the immunohistochemical markers for axonal injury beta-amyloid precursor protein (β-APP) (Chemicron 22C11 used at dilution of 1:5000) and counterstained with Meyer's hematoxylin. Every field of the slides was examined by a blinded reviewer at scanning power (5–10× magnification). Specific fields were examined at 20–40× magnification. Locations of axonal injury, subarachnoid and parenchymal hemorrhage, and cell death were noted on digital photographs of the coronal sections. Total brain area was measured by tracing the brain area in scaled digital images of each of the slices (ImageJ), and summing the results from each slice. Established infarcts on H&E staining were identified by changes in staining intensity, and ischemic neurons, characterized by cell shrinkage and cytoplasmic eosinophilia 17. Regions of β–APP reactivity and infarction were noted by the neuropathologist on these images and the locations of white matter damage and infarction were traced in each slice using the same procedure to determine total area. Total and injured areas were multiplied by section thickness to determine total and injured brain volumes.
Statistical Analysis
Physiological and arterial blood gas data were analyzed across groups, and time using two-way ANOVA tests and Tukey-Kramer tests were used for post hoc analysis. Neuromonitoring parameters (ICP, PbtO2, microdialysis) were analyzed using two-way ANOVA tests. Again, Tukey-Kramer tests were utilized with significance, defined as P < 0.05. Linear mixed-effects model was used to obtain an estimate of the correlation between two variables when multiple measurements were available on the variables of interest 18. Neuropathology comparisons between injured and shams were analyzed using Student's t-test. All values are expressed as mean ± the standard error of the mean.
RESULTS
Physiological data
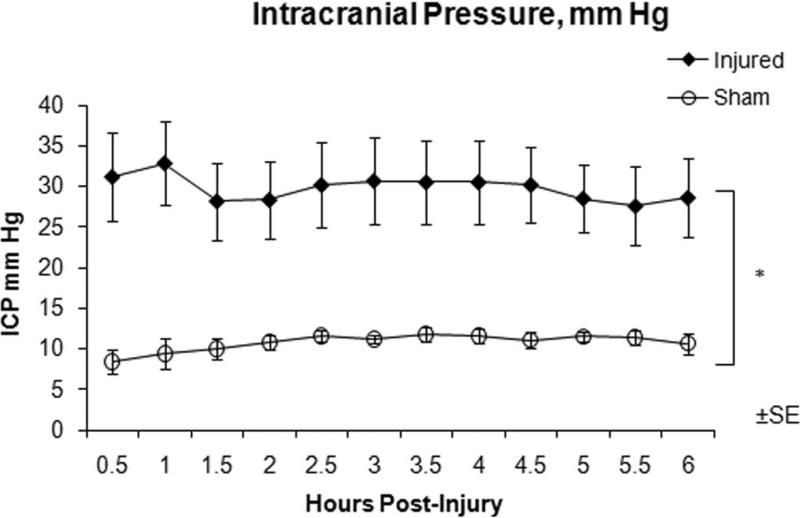
Physiological and arterial blood gas data for INJ and SHAM are shown in Tables 1 and 2, respectively. INJ had significantly higher serum lactate levels at post-injury hour 3 and 6, although these levels were still within normal range for immature swine 19. Although end tidal CO2 was maintained between 35-40 mm Hg, arterial PaCO2 levels were observed to be higher than the corresponding end tidal CO2 but no significant difference in arterial pH or PaCO2 between the two groups was observed. Intracranial pressure remained significantly elevated in INJ compared to SHAM throughout the post-injury period (Figure 2). CPP was significantly lower in INJ compared to SHAM one hour after injury and remained lower for the entire 6 hours. Average unconscious times for INJ were 37.6 ± 15.1 and 12.2 ± 1.1 minutes for SHAM, which are longer when compared to our previous studies but this is most likely attributable to a change in anesthetic regimen with the addition of fentanyl.
Table 1.
Physiological data pre-injury, 1 hour, 3 hours, and 6 hours post-injury.
| Parameter | Injured | Sham |
|---|---|---|
| Heart Rate (beats/min) | ||
| Baseline | 101.3 ± 8.0 | 109.0 ± 9.3 |
| 1 hour | 121.3 ± 9.6* | 90.8 ± 7.3 |
| 3 hour | 140.5 ± 7.8* | 112.4 ± 9.4 |
| 6 hour | 135.3 ± 12.2 | 123.6 ± 8.1 |
| MAP (mm Hg) | ||
| Baseline | 70.3 ± 1.4 | 75.0 ± 4.1 |
| 1 hour | 70.0 ± 5.9 | 72.0 ± 2.6 |
| 3 hour | 54.5 ± 5.8 | 63.4 ± 1.7 |
| 6 hour | 44.1 ± 6.0* | 63.2 ± 2.7 |
| CPP (mm Hg) | ||
| 1 hour | 37.2 ± 9.8* | 63.8 ± 2.7 |
| 3 hour | 24.7 ± 9.6* | 49.8 ± 3.6 |
| 6 hour | 20.8 ± 11.1* | 54.0 ± 2.0 |
| Temperature (°C) | ||
| Baseline | 36.8 ± 0.2 | 36.9 ± 0.4 |
| 1 hour | 37.3 ± 0.3 | 37.1 ± 0.5 |
| 3 hour | 37.7 ± 0.3 | 37.3 ± 0.5 |
| 6 hour | 38.1 ± 0.3 | 37.4 ± 0.5 |
(MAP) mean arterial pressure, (CPP) cerebral perfusion pressure. Note: There are no pre-injury CPP values as ICP was not measured until after injury.
denotes statistical difference compared to SHAM, P< 0.05.
Table 2.
Arterial blood gas data pre-injury, 1 hour, 3 hours and 6 hours post-injury.
| Parameter | Injured | Sham |
|---|---|---|
| pH | ||
| Baseline | 7.53 ± 0.02 | 7.52 ± 0.02 |
| 1 hour | 7.47 ± 0.03 | 7.46 ± 0.03 |
| 3 hour | 7.46 ± 0.02 | 7.43 ± 0.02 |
| 6 hour | 7.45 ± 0.03 | 7.45 ± 0.01 |
| PaCO2 (mm Hg) | ||
| Baseline | 43.7 ± 3.3 | 43.7 ± 2.7 |
| 1 hour | 50.1 ± 3.9 | 46.9 ± 2.0 |
| 3 hour | 51.4 ± 6.1 | 50.8 ± 0.5 |
| 6 hour | 48.5 ± 2.5 | 51.2 ± 0.5 |
| PaO2 (mm Hg) | ||
| Baseline | 150.9 ± 11.5 | 128.4 ± 14.5 |
| 1 hour | 135.3 ± 10.4 | 128.35 ± 11.9 |
| 3 hour | 147.6 ± 12.7 | 120.9 ± 12.7 |
| 6 hour | 139.9 ± 11.0 | 123.7 ± 6.8 |
| Lactate (mmol/L) | ||
| Baseline | 1.08 ± 0.22 | 0.775 ± 0.04 |
| 1 hour | 1.23 ± 0.25 | 0.95 ± 0.11 |
| 3 hour | 1.22 ± 0.17* | 0.65 ± 0.07 |
| 6 hour | 0.9 ± 0.14* | 0.6 ± 0.03 |
| Sodium (mmol/L) | ||
| Baseline | 139.8 ± 2.2 | 140.6 ± 1.2 |
| 1 hour | 140.3 ± 1.5 | 141.1 ± 1.6 |
| 3 hour | 141.6 ± 1.7 | 140.2 ± 0.8 |
| 6 hour | 139.7 ± 1.3 | 141.2 ± 1.3 |
| Glucose (mg/dL) | ||
| Baseline | 82.2 ± 5.8 | 83.3 ± 10.3 |
| 1 hour | 102.0 ± 11.4 | 88.7 ± 6.8 |
| 3 hour | 92.8 ± 11.9 | 82.3 ± 5.7 |
| 6 hour | 89.2 ± 9.2 | 76.0 ± 2.3 |
denotes statistical difference compared to SHAM, P < 0.05.
Figure 2.
Intracranial pressure over the 6 hour post-injury period measured via fiber-optic intracranial pressure monitor for injured (closed diamonds) and sham (open circles) animals. * Statistical difference (P < 0.02).
Brain tissue oxygen tension
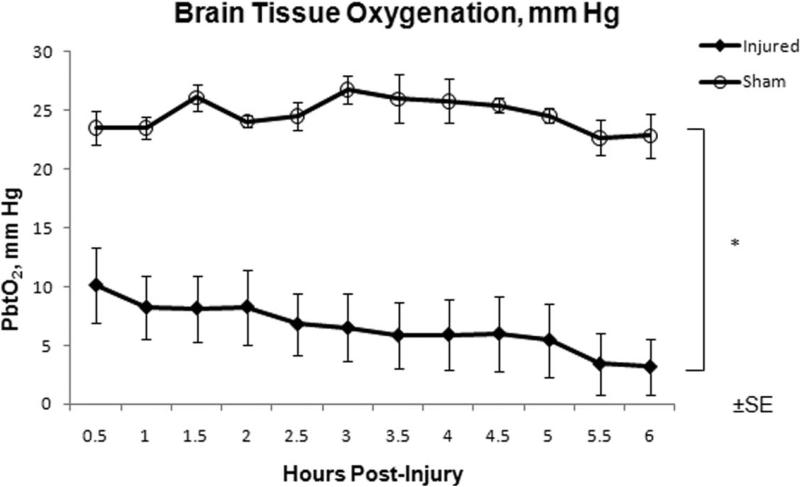
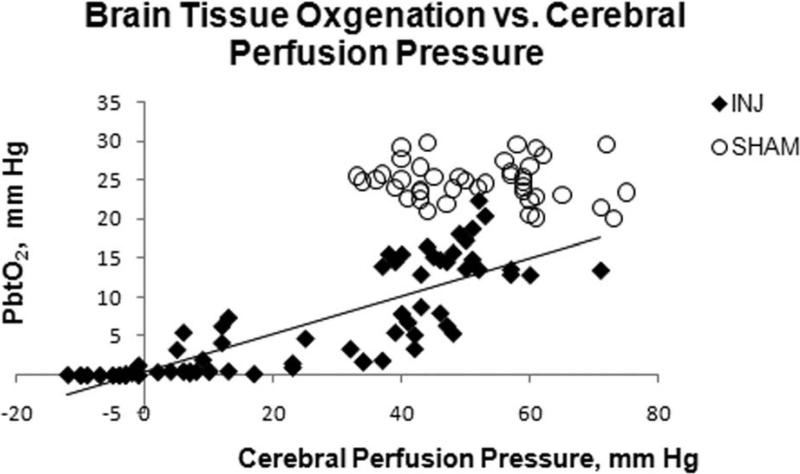
PbtO2 in the frontal lobe was significantly higher in SHAM compared to INJ throughout the 6 hour post-injury period (Figure 3). There was no significant difference in arterial PaO2 between the groups (Table 2) throughout the six hour post injury period, which could have contributed to this difference. PbtO2 in INJ had a linear correlation with increasing CPP (0.83, P < 0.05), but not in SHAM (Figure 4). Sub analysis of PbtO2 when CPP was greater than 40 mm Hg in both groups revealed significantly higher PbtO2 levels in SHAM compared to INJ (24.8 ± 0.4 mm Hg vs. 12.9 ± 0.9 mm Hg, P < 0.05).
Figure 3.
Brain tissue oxygen over the 6 hour post-injury period measured in the frontal lobe for injured (closed diamonds) and sham (open circles) animals. * Statistical difference (P < 0.05).
Figure 4.
Brain tissue oxygen values in injured animals (closed diamonds) strongly correlated with cerebral perfusion pressure (R2 = 0.6775). No correlation was observed in sham animals (open circles).
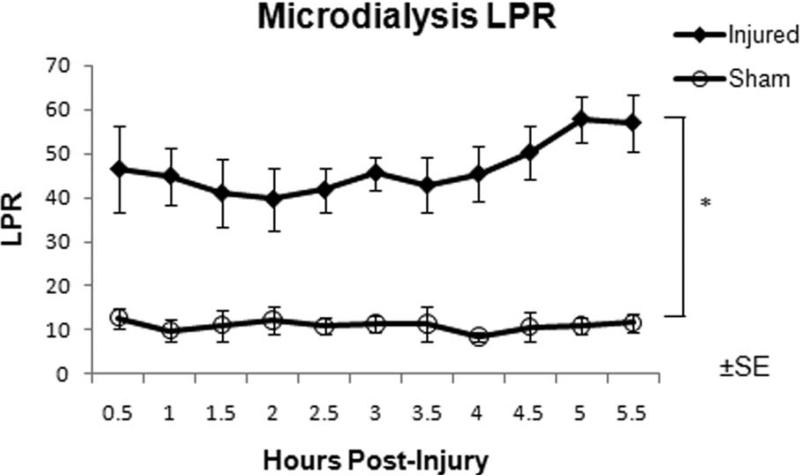
Lactate-pyruvate ratio (LPR)
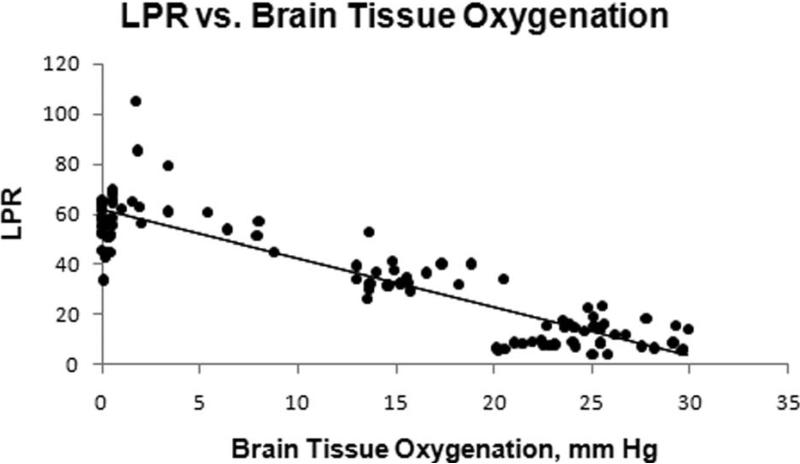
Microdialysis samples were collected every 30 minutes with the initial collection commencing 30 minutes after injury. LPR were significantly elevated in INJ compared to SHAM throughout the post-injury period (Figure 5). LPR and PbtO2 from the contralateral frontal lobe had a strong linear correlation in both SHAM and INJ (-0.92, P < 0.05, Figure 6), although this correlation decreased if only INJ values were included (-0.81, P < 0.05 ).
Figure 5.
Microdialysis LPR over the 6 hour pos-injury period measured in the frontal lobe for injured (closed diamonds) and sham (open circles) animals. Samples were collected every 30 minutes. * Statistical difference (P < 0.05).
Figure 6.
A strong correlation (R2 = 0.8012) between PbtO2 and LPR in the contralateral frontal lobe was observed in both INJ and SHAM.
Neuropathology
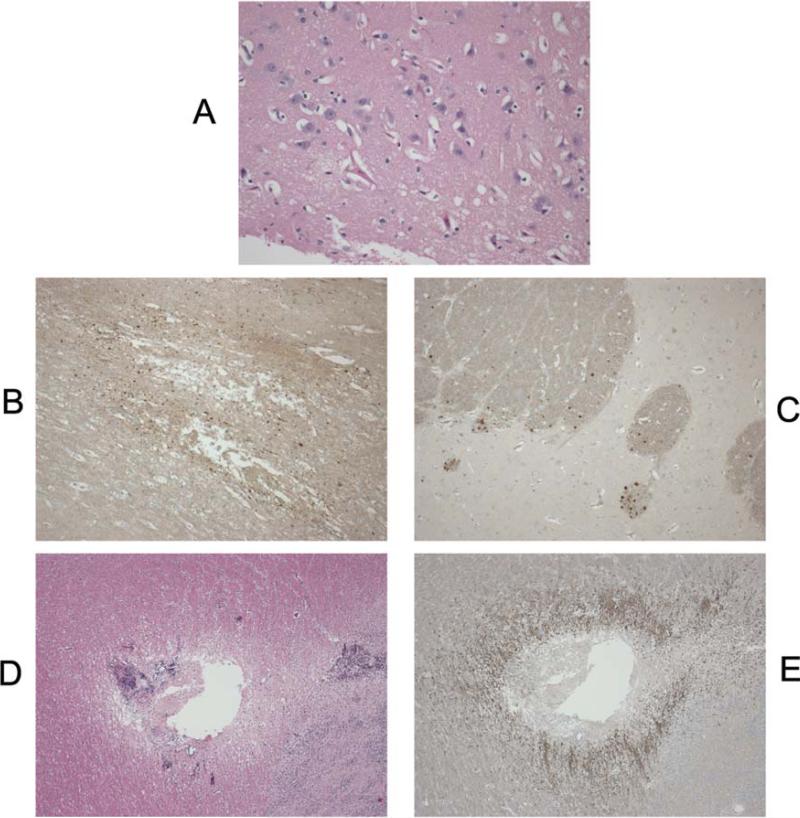
Regions of injury were predominantly in the frontal and parietal lobes. Axonal injury volume at 6 hours post injury as determined by β-APP immunohistochemistry was significantly higher in INJ compared to SHAM (0.38% for INJ vs. 0.05% for SHAM, p < 0.02). Infarct volume determined by H&E was also significantly larger in INJ compared to SHAM (9.83% for INJ vs. 0.04% for SHAM, P < 0.05). In SHAM, axonal injury and infarct were only observed in the frontal lobes at probe insertion sites (Figure 7).
Figure 7.
(A) H&E staining of area of ischemic injury in an injured animal (20x magnification). The damaged neurons have shrunken nuclei and cytoplasm, and there is fine vacuolation of the neutrophils. Examples of β-APP: staining ischemic injury (B) and traumatic injury (C) at 10x magnification. Note how the staining respects the white matter bundle orientation in (C) compared to (B). Ischemic injury pattern around a subcortical lesion in sham animal related to probe placement (D) H&E and (E) β-APP at 4x magnification.
Correlation of intracranial monitoring with neuropathology
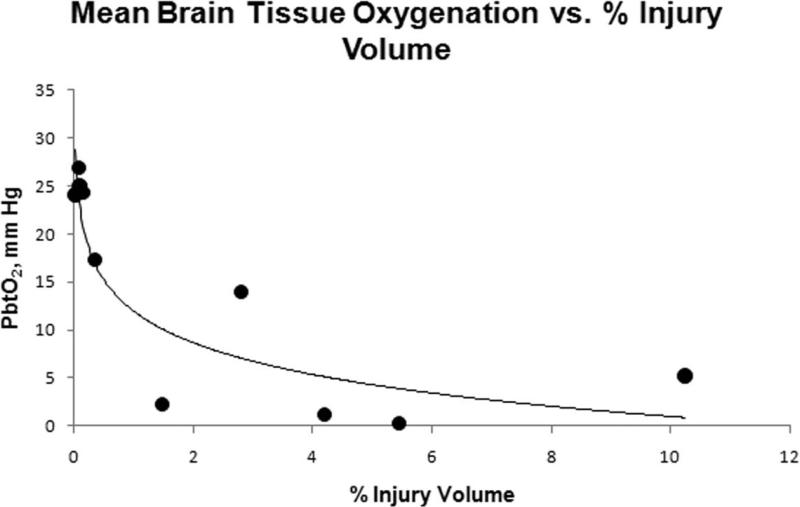
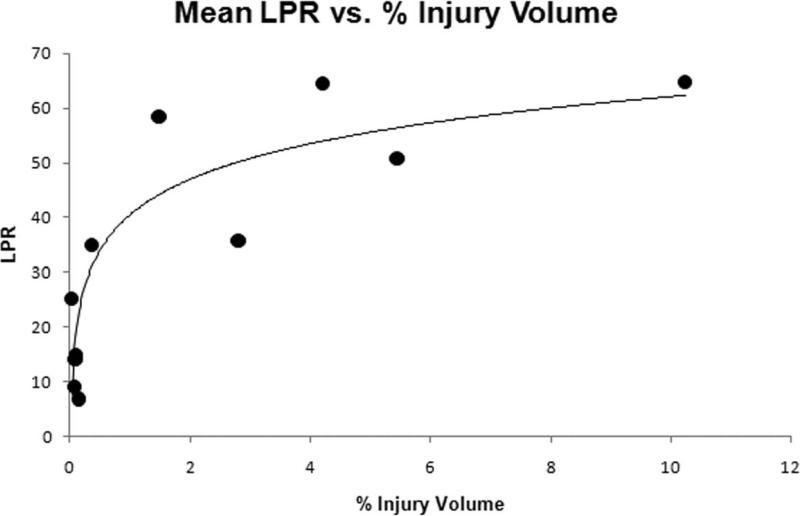
Two-way ANOVA analysis of both PbtO2 and LPR did not demonstrate a significant effect of time post injury, so values for each animal were averaged over the 6 hour post-injury period to assess correlation with injury volume. Average PbtO2 in both INJ and SHAM had a strong inverse correlation with total injury volume (infarct + axonal injury volume) (-0.85, P < 0.005) (Figure 8). Average LPR demonstrated a strong correlation with total injury (0.77, P < 0.005) (Figure 9). Axonal injury volume alone also had similar strong correlations with average PbtO2 (-0.81, P < 0.005) and average LPR (0.84, P < 0.005). When injured animals were analyzed alone, total injury volume had weaker correlations with average PbtO2 (-0.61) and average LPR (0.65) and did not reach statistical significance.
Figure 8.
Mean PbtO2 over the 6 hour period and % injury volume for each animal.
Figure 9.
Mean LPR over the 6 hour period and % injury volume for each animal
DISCUSSION
Multi-parametric neuromonitoring in TBI has been introduced into pediatric intensive care units as a way to detect changes in the complex pathophysiological responses that occur following traumatic brain injury. But what still remains unclear is how to interpret and respond to changes in multi-parametric monitoring to improve pediatric TBI outcomes. The heterogeneity of mechanisms and phenotypes in pediatric TBI, along with challenges in monitor probe placement, and age dependent changes in responses, have hindered utilization of this monitoring data in clinical decision-making 20-22. A controlled animal model can be used as a standardized platform for pediatric TBI neurocritical care to correlate routinely measured pathophysiological responses with multi-parametric neuromonitoring data, and study new therapies and interventions before embarking on large multi-center clinical trials.
This report describes the development of an immature swine model of pediatric neurocritical care using our previously published model of nonimpact inertial head injury 12, 14. Previous investigators have used neuromonitoring in adult swine models of TBI 9, 11. To our knowledge, this is the first report of a large animal model of pediatric neurocritical care with multi-parametric neuromonitoring. This large animal model can be fully instrumented with central venous access and arterial access similar to the critically ill pediatric TBI patient, allowing us to simulate the pediatric intensive care environment. Our model has several unique features, including brain size, injury distribution, and large rises in ICP. First, we selected 4 week old piglets (brain weight 65 grams) to facilitate the placement of multiple intracranial probes. Second, the wide distribution of hemorrhage and axonal injury allows for region-to-region comparisons across monitored parameters, which is not possible with focal injuries. We previously demonstrated the diffuse nature of our injury model with widespread axonal injury in the frontal lobes at 6 hours, 5 days and 12 days post injury 12-15. The strong correlation of PbtO2 and LPR values (Figure 6) from opposite frontal lobes supports these observations. SHAM ICP values were observed to be similar to non-injured pediatric patients, supporting the clinical relevance of our animal model 23. ICP was markedly elevated after injury and sustained elevations were observed throughout the 6 hour post-injury observation period reflecting the severity of the injury in our animal model. This intracranial hypertension following injury is unique to our closed head injury model. Armstead et al have utilized a fluid percussion injury (FPI) in a piglet model with ICP monitoring and demonstrated increases in ICP compared to SHAM, but never achieved levels consistent with intracranial hypertension that would warrant treatment in the clinical setting 24. The sustained, stable elevation in ICP we observe provides us the opportunity to study various post-injury delays in administration of interventions or therapies, mimicking the clinical interval between injury, stabilization, and finally transfer to a trauma center or pediatric intensive care unit.
Clinical studies in adult and pediatric TBI patients have proposed that PbtO2 levels of less than 20 mm Hg in white matter are associated with cerebral ischemia 25, 26. In addition, PbtO2 has been observed to correlate with cerebral blood flow in severe head injury patients, and thought to provide a measure of substrate delivery to the region of interest 27. In our model, we find that PbtO2 levels in injured animals were observed to be stable, and on average less than 15 mm Hg throughout the post-injury period. Because injury volume at 6 hours post-injury correlated well with PbtO2, we can utilize PbtO2 in our injury model as a serial secondary endpoint when studying new responses to interventions or therapies. Interestingly, CPP and PbtO2 in INJ had a linear relationship, whereas no correlation was observed in SHAM, perhaps attributable to disruptions in autoregulation in the injured animals. This CPP dependence of PbtO2 has been observed in children with severe traumatic brain injury 28. However, our observation would also be consistent with a linear relationship between CPP and CBF at the low CPPs we observed in injured animals. Further studies exploring a broader range of CPPs after injury in our model are currently ongoing. PbtO in injured animals at CPP > 40 mm Hg was significantly lower compared to SHAM PbtO2 at CPP > 40 mm Hg. Pediatric traumatic brain injury guidelines currently recommend maintaining CPP above a threshold of 40 mm Hg. A CPP of 40 mm Hg based on our PbtO2 values may not be sufficient to reduce the incidence of cerebral ischemia early after injury. Furthermore, ICP monitoring may not occur for several hours in the pediatric head injured patient depending on transport time and the capabilities of the initial receiving facility. Our model highlights that aggressive and rapid cerebral resuscitation may be needed to prevent cerebral ischemia in severe pediatric TBI patients. Future studies are planned to investigate what effect aggressive manipulation of CPP early after closed head injury has on physiologic variables as well as neuropathology in our model.
Microdialysis permits sampling and analysis of lactate and pyruvate in the cerebral extracellular fluid. The LPR of the interstitial fluid reflects cellular glucose delivery, utilization, and the extent of anaerobic glycolysis 29. In our model, we were interested in investigating cellular metabolic crisis utilizing LPR and determining if LPR correlates with injury severity and neuropathology. LPR was observed to be significantly elevated in INJ compared to SHAM. LPR correlated well with PbtO2 measured in the other frontal lobe, which we attribute to the diffuse nature of the injury. PbtO2 from the contralateral lobe of less than 10 mm Hg were associated with LPR values > 40, which is consistent with previous studies in animals and humans 29. It is unclear why the relationship of PbtO2 to LPR was not as strong if INJ animals were only included, but may be partly due to the variability in LPR values at very low levels of PbtO2 (Figure 6). CPP did not correlate as strongly with LPR as it did with PbtO2. This may be due to the fact that cerebral microdialysis reflects metabolic crisis over the sample interval and in this study we did not record continuous CPP values. Because average LPR correlated well with injury volumes assessed at 6 hours, we can utilize microdialysis LPR as another serial nonterminal secondary marker in our injury model for neuropathology while studying interventions during the acute post-injury period.
Our post-TBI neurocritical care animal model has some limitations. First, neuromonitoring was only performed for six hours after injury and, thus, did not capture the entire pathophysiological response following pediatric TBI. Extensive resources and personnel are needed to extend the neurocritical care period further to recover animals for neurobehavioral functional testing 13, 30. Second, our injury model of TBI produces widespread axonal injury, and subarachnoid and subdural hemorrhage, but does not simulate the full spectrum of pediatric brain injury, most importantly focal injuries. One of the challenges with cerebral microdialysis and brain tissue oxygenation for TBI is probe placement, especially in focal injury. These intracranial probes can only report regional changes in LPR and PbtO2 and are therefore influenced by probe location 29, 31. Should probes be placed directly into injured tissues, the “penumbra”, or in brain tissue thought to be uninjured? How does the probe location in relation to injury affect interpretation of multi-parametric neuromonitoring? Current consensus statements recommend placement in the pericontusional area for focal injuries and in the frontal lobe for diffuse injuries 32. We chose probe placement in the frontal lobes due to the diffuse injury that is produced in our model. Probe placement in our model did create some injury as demonstrated with axonal injury and infarct volumes in SHAM, but the total volumes were quite small compared to injury volumes in INJ and these focal injuries due to probe placement did not result in alterations in intracranial physiologic monitoring in our SHAM animals. A third limitation of this study is that we utilized LPR and PbtO2 as surrogates for substrate delivery, but we did not measure cerebral blood flow (CBF). Clinical studies have demonstrated that following pediatric TBI, there is a significant drop in CBF 33. We have recently begun utilizing a novel thermal diffusion flowmetry probe (Hemedex, Cambridge, MA) in our model that has the ability to continuously measure real time CBF in a region of interest 34, 35.
CONCLUSION
We present our large animal model of pediatric neurocritical care with multi-parametric neuromonitoring following nonimpact inertial head injury that produces pathophysiological responses similar to pediatric TBI. We used important characteristics of the piglet brain to create a unique translational model to develop promising new therapeutics to treat pediatric traumatic brain injury in the acute care setting. This translational model bridges a vital gap in knowledge between successful TBI studies in small animal models and the failure of clinical trials in the TBI population. By translating promising therapeutics and interventions from rodent to our porcine model, and providing state of the art neurocritical care monitoring, we hope to generate preclinical large animal data to build a practical, conceptual bridge to pediatric clinical trials.
ACKNOWLEDGEMENTS
The authors would like to thank Suzanne Frangos for her technical assistance in analyzing microdialysis samples.
Sources of Support
NIH grant K08-NS064051 (SHF)
The Endowed Chair in Critical Care Medicine, The Children's Hospital of Philadelphia (MAH)
NIH grant R01-NS39679 (SSM)
Biography
Friess et al describe a large animal model of pediatric TBI using rapid axial head rotation without impact in 4 week-old piglets. They studied 11 animals with systemic and intracranial physiological monitoring including intracranial pressure (ICP), brain tissue oxygen tension (PbtO2), and cerebral microdialysis with monitoring until 6 hours after injury. They found a substantial rise in intracranial pressure following injury compared with controls with peak ICP around 30 mm Hg. They also found a substantial decrease in brain tissue oxygen tension and a rise in the lactate to pyruvate ratio in injured animals compared to controls. Injury volume in the brain correlated with PbtO2 and lactate to pyruvate ratio (LPR). The authors are to be commended for describing this translational model that is a clinically relevant model of pediatric TBI. Importantly, in the described model a substantial elevation of ICP occurs. This is a significant accomplishment, as many previous large animal models of TBI have not succeeded in raising ICP beyond the clinically significant threshold of 20-25 mm Hg.
The current protocol does have some limitations. Because the injury led to a substantial rise in ICP, the mean cerebral perfusion pressure (CPP) in injured animals was quite low, 25 and 21 mm Hg, at 3 and 6 hours post-injury, respectively. This is a much lower CPP than would likely be tolerated in a pediatric ICU setting even relatively early after injury. This may limit the clinical relevance and in part account for the large injury volumes observed in the injured animals. In addition, as pointed out by the authors, the linear relationship between PbtO2 and CPP in injured animals may not reflect a loss of autoregulation, but simply result from the fact that at such low CPP the normal pressure limits of autoregulation have been exceeded. At such low CPP, a linear relationship between CPP and cerebral blood flow is expected and could account for the observed relationship between PbtO2 and CPP. These issues will need to be investigated further in future studies. What is clear is that this model can serve as a basis for further clinically relevant studies of pediatric TBI. This work emphasizes the importance of translational models that allow for intensive physiological and cerebral multimodality monitoring in a setting that mimics the modern intensive care unit. It is hoped that future work will use this platform to investigate innovative treatment strategies that can lead to improved outcomes in pediatric TBI.
Guy Rosenthal
Jerusalem, Israel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007 Feb;119(2):345–360. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 2.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4:S1–75. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 3.Duhaime AC. Why are clinical trials in pediatric head injury so difficult? Pediatr Crit Care Med. 2007 Jan;8(1):71. doi: 10.1097/01.PCC.0000253552.60687.AC. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg H, Ichord R, Palmer C, Yager JY, Vannucci SJ. Animal Models of Developmental Brain Injury: Relevance to Human Disease. Dev Neurosci. 2002;24:364–366. doi: 10.1159/000069040. [DOI] [PubMed] [Google Scholar]
- 5.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8(1):30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 6.Durham SR, Raghupathi R, Helfaer MA, Marwaha S, Duhaime AC. Age-related differences in acute physiologic response to focal traumatic brain injury in piglets. Pediatr Neurosurg. 2000 Aug;33(2):76–82. doi: 10.1159/000028980. [DOI] [PubMed] [Google Scholar]
- 7.Flynn TJ. Developmental changes of myelin-related lipids in brain of miniature swine. Neurochem Res. 1984 Jul;9(7):935–945. doi: 10.1007/BF00964525. [DOI] [PubMed] [Google Scholar]
- 8.Wootton R, Flecknell PA, John M. Accurate measurement of cerebral metabolism in the conscious, unrestrained neonatal piglet. I. Blood flow. Biol Neonate. 1982;41(5-6):209–220. doi: 10.1159/000241553. [DOI] [PubMed] [Google Scholar]
- 9.Alessandri B, Heimann A, Filippi R, Kopacz L, Kempski O. Moderate controlled cortical contusion in pigs: effects on multi-parametric neuromonitoring and clinical relevance. J Neurotrauma. 2003 Dec;20(12):1293–1305. doi: 10.1089/089771503322686094. [DOI] [PubMed] [Google Scholar]
- 10.Timaru-Kast R, Meissner A, Heimann A, Hoelper B, Kempski O, Alessandri B. Acute subdural hematoma in pigs: role of volume on multiparametric neuromonitoring and histology. J Neurotrauma. 2008 Sep;25(9):1107–1119. doi: 10.1089/neu.2008.0517. [DOI] [PubMed] [Google Scholar]
- 11.Manley GT, Rosenthal G, Lam M, et al. Controlled cortical impact in swine: pathophysiology and biomechanics. J Neurotrauma. 2006 Feb;23(2):128–139. doi: 10.1089/neu.2006.23.128. [DOI] [PubMed] [Google Scholar]
- 12.Friess SH, Ichord RN, Owens K, et al. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp Neurol. 2007 Mar;204(1):234–243. doi: 10.1016/j.expneurol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friess SH, Ichord RN, Ralston J, et al. Repeated traumatic brain injury affects composite cognitive function in piglets. J Neurotrauma. 2009 Mar 10; doi: 10.1089/neu.2008.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghupathi R, Margulies SS. Traumatic axonal injury after closed head injury in the neonatal pig. J Neurotrauma. 2002;19:843–853. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- 15.Raghupathi R, Mehr MF, Helfaer MA, Margulies SS. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J Neurotrauma. 2004 Mar;21(3):307–316. doi: 10.1089/089771504322972095. [DOI] [PubMed] [Google Scholar]
- 16.Duhaime AC, Margulies SS, Durham SR, et al. Maturation-dependent response of the piglet brain to scaled cortical impact. J Neurosurg. 2000 Sep;93(3):455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- 17.Eucker SA, Smith C, Ralston J, Friess SH, Margulies SS. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp Neurol. 2010 Sep 25; doi: 10.1016/j.expneurol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam M, Webb CA, O'Donnell DE. Linear mixed-effects model was used to obtain an estimate of the correlation between two variables when multiple measurements are available on the variables of interest. American Statistical Association, Proceedings of the Biometric Section. 1999:213–218. [Google Scholar]
- 19.Hannon JP, Bossone CA, Wade CE. Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci. 1990 May;40(3):293–298. [PubMed] [Google Scholar]
- 20.Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007 Jul;23(3):507–538. doi: 10.1016/j.ccc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 2: Relationship with clinical, physiological, and treatment factors. Childs Nerv Syst. 2009 Oct;25(10):1335–1343. doi: 10.1007/s00381-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 22.Charalambides C, Sgouros S, Sakas D. Intracerebral microdialysis in children. Childs Nerv Syst. 2010 Feb;26(2):215–220. doi: 10.1007/s00381-009-1031-3. [DOI] [PubMed] [Google Scholar]
- 23.Blomquist HK, Sundin S, Ekstedt J. Cerebrospinal fluid hydrodynamic studies in children. J Neurol Neurosurg Psychiatry. 1986 May;49(5):536–548. doi: 10.1136/jnnp.49.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstead WM, Kiessling JW, Kofke WA, Vavilala MS. Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit Care Med. 2010 Sep;38(9):1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doppenberg EM, Zauner A, Watson JC, Bullock R. Determination of the ischemic threshold for brain oxygen tension. Acta Neurochir Suppl. 1998;71:166–169. doi: 10.1007/978-3-7091-6475-4_48. [DOI] [PubMed] [Google Scholar]
- 26.Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998 Sep;26(9):1576–1581. doi: 10.1097/00003246-199809000-00029. [DOI] [PubMed] [Google Scholar]
- 27.Doppenberg EM, Zauner A, Bullock R, Ward JD, Fatouros PP, Young HF. Correlations between brain tissue oxygen tension, carbon dioxide tension, pH, and cerebral blood flow--a better way of monitoring the severely injured brain? Surg Neurol. 1998 Jun;49(6):650–654. doi: 10.1016/s0090-3019(97)00355-8. [DOI] [PubMed] [Google Scholar]
- 28.Figaji AA, Zwane E, Fieggen AG, et al. Pressure autoregulation, intracranial pressure, and brain tissue oxygenation in children with severe traumatic brain injury. J Neurosurg Pediatr. 2009 Nov;4(5):420–428. doi: 10.3171/2009.6.PEDS096. [DOI] [PubMed] [Google Scholar]
- 29.Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005 Jan;22(1):3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- 30.Hanneman SK, Clubb FJ, Jr., McKay K, Costas G. Feasibility of a porcine adult intensive care model. Comp Med. 2004 Feb;54(1):36–43. [PubMed] [Google Scholar]
- 31.Stiefel MF, Udoetuk JD, Storm PB, et al. Brain tissue oxygen monitoring in pediatric patients with severe traumatic brain injury. J Neurosurg. 2006 Oct;105(4 Suppl):281–286. doi: 10.3171/ped.2006.105.4.281. [DOI] [PubMed] [Google Scholar]
- 32.Bellander BM, Cantais E, Enblad P, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004 Dec;30(12):2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- 33.Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: A preliminary report. Pediatric Neurosurgery. 1997;26:200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med. 2007 Jul;33(7):1263–1271. doi: 10.1007/s00134-007-0678-z. [DOI] [PubMed] [Google Scholar]
- 35.Vajkoczy P, Roth H, Horn P, et al. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg. 2000 Aug;93(2):265–274. doi: 10.3171/jns.2000.93.2.0265. [DOI] [PubMed] [Google Scholar]