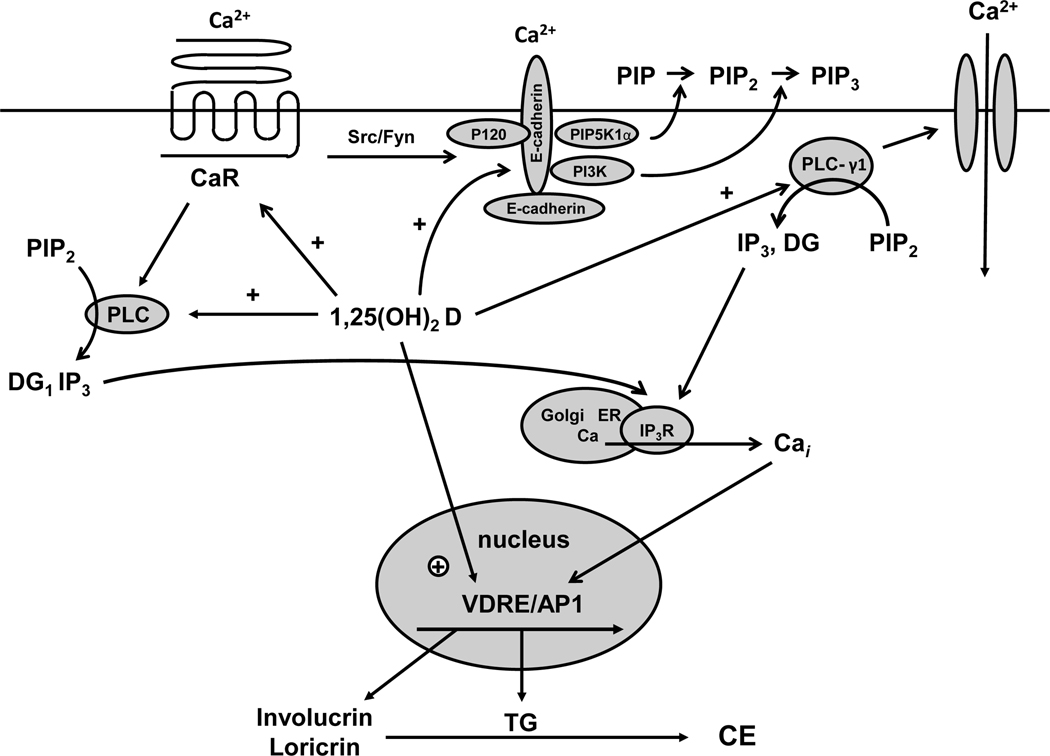
Figure 3. Calcium and 1,25(OH)2D interactions in the regulation of keratinocyte differentiation.
Critical steps in calcium induced keratinocyte differentiation involve activation of the calcium sensing receptor (CaR) and formation of the E-cadherin complex at the membrane. This complex includes a number of catenins and phospholipid modifying enzymes (PI3K, PIP5K1α) that are critical for subsequent differentiation events. In particular the activation of phospholipase C-γ1 (PLC-γ1), the enzyme largely responsible for maintaining increased levels of intracellular calcium (Cai) through its effects on both plasma membrane calcium channels and in the production of IP3 from PIP2 for stimulation of calcium release from intracellular stores, requires the formation of the E-cadherin complex. Hydrolysis of PIP2 by PLC-γ1 also leads to diacylglycerol production (DG) and activation of protein kinases C, that also play an important role in keratinocyte differentiation. Within hours of the calcium switch keratinocytes change from making the basal keratins K5 and K14 to making keratins K1 and K10 followed, subsequently, by increased levels of profilaggrin, involucrin and loricrin. Loricrin, involucrin and other proteins are cross linked into the insoluble cornified envelope by the calcium sensitive, membrane bound form of transglutaminase, which like involucrin and loricrin increases within 24 hours after the calcium switch. The induction of these proteins represents a genomic action (likely indirect) of calcium as indicated by a calcium induced increase in mRNA levels and transcription rates. The CaR by regulating phospholipase C (PLC) activity controls the production of inositol tris phosphate (IP3) and diacyl glycerol (DG). PLC-β is activated directly by CaR via a G protein coupled mechanism, whereas PLC-γ1 is activated by phosphatidyl inositol tris phosphate (PIP3), levels that are maintained in the membrane by phosphatidyl inositol 3 kinase (PI3K) bound to the E-cadherin complex. CaR regulates E-cadherin complex formation through src/fyn tyrosine kinases that phosphorylate the catenins and PI3K essential for their binding to E-cadherin. Extracellular calcium (Cao) activates these processes through the CaR and by stabilizing E-cadherin membrane localization. 1,25(OH)2D modulates calcium regulated differentiation at several steps. First, 1,25(OH)2D increases CaR expression, thus making the cell more responsive to calcium. Second, 1,25(OH)2D induces all the PLCs, as does calcium, again increasing the responsiveness of the cell to calcium. Third, the VDR is required for calcium induced formation of the E-cadherin complex in the membrane, in part through induction of E-cadherin as well as CaR. Finally, 1,25(OH)2D induces the transcription of genes such as involucrin and transglutaminase and possibly the other differentiation markers in addition to PLC and CaR.