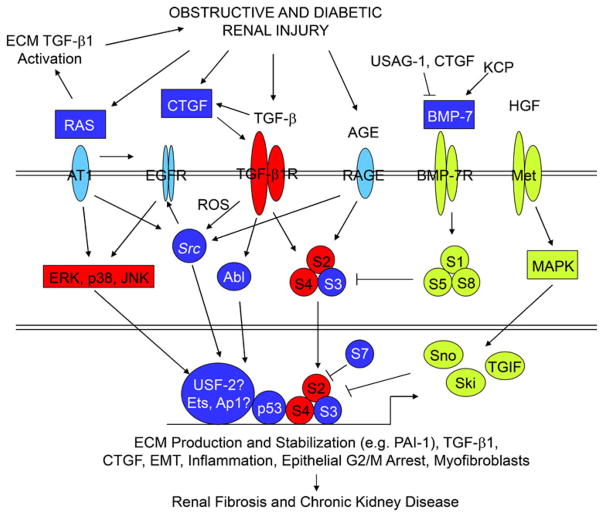
Fig. 3.
TGFβ1/SMAD3 signaling as an integrator of fibrotic mechanisms in the kidney. The pathophysiology of obstructive and diabetic renal fibrosis involves the activation of multi-component receptor-initiated signaling events. Representation of the individual interacting systems discussed in this review. Whereas unique elements are found in each, TGF-β1/SMAD2/3 signaling appears to integrate downstream effectors of these collateral pathways, in certain circumstances, to promote a profibrotic microenvironment. Persistent TGF-β1 expression and SMAD2/3 phosphorylation consistently accompany progression of chronic renal disease in animal models and humans. Expression of negative regulatory TGF-β1/SMAD pathway elements (inhibitory SMAD-7, Ski, Sno) are reduced in the context of tissue fibrosis, thus, further amplifying TGF-β1-initiated fibrogenic processes (e.g., ECM synthesis, EMT, inflammation, CNN2 [previously designated connective tissue growth factor] and PAI-1 induction, and myofibroblast differentiation/persistence). Moreover, both the expression and signaling ability of negative effectors of TGF-β1 signaling (e.g., BMP-7, hepatocyte growth factor [HGF]) are also suppressed by a variety of mechanisms (detailed in the text) facilitating maintenance of TGF-β1 signaling in the injured tissue leading to excessive deposition of ECM components, disruption of renal parenchyma, and progressive loss of kidney function