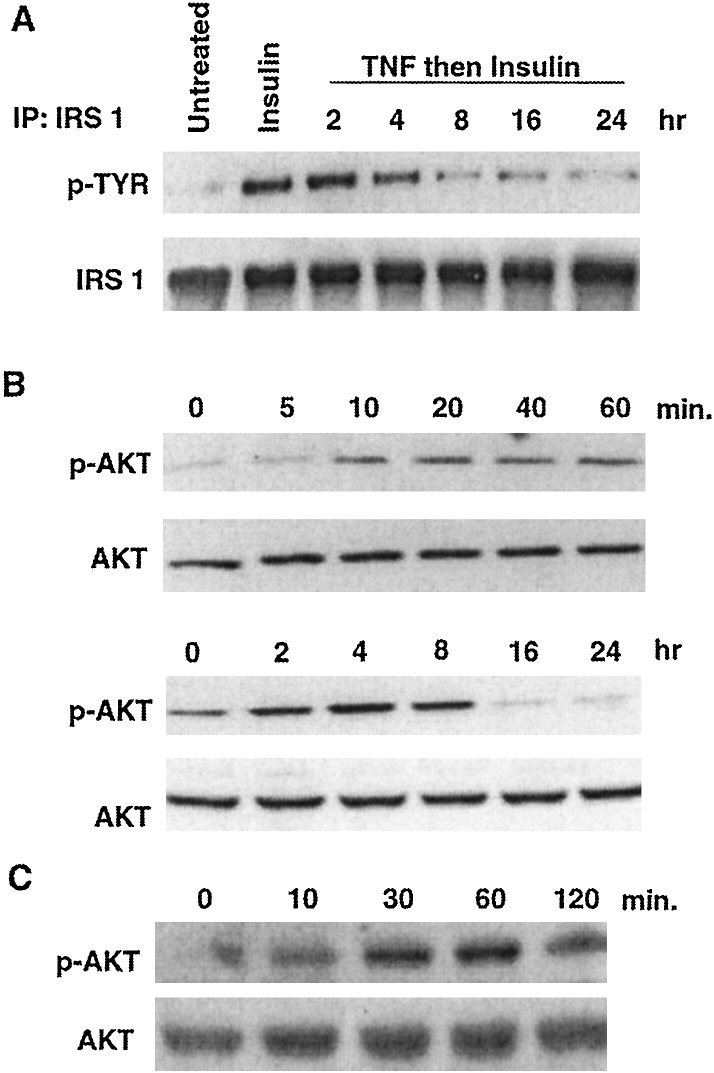
Figure 1.
TNF inhibits tyrosine phosphorylation of IRS-1 and activates Akt. (A) Serum-starved NIH 3T3 fibroblasts were incubated with medium, insulin (10 nM, 5 min), or TNF (10 nM) for various times and then insulin. After immunoprecipitation (IP) of IRS-1 a Western blot was probed with an antibody to phosphotyrosine and then with anti-IRS-1 to demonstrate fractionation of equal amounts of protein from cell lysates. (B) Serum-starved NIH 3T3 cells were incubated with 10 nM TNF for 0–60 min (Upper) and 0–24 h (Lower). Cell lysates were fractionated on 10% polyacrylamide gels and Western blots were probed with an antibody directed against phospho-Akt (Upper), stripped, and reprobed with an anti-Akt antibody (Lower). (C) Serum-starved myotubes were incubated with 10 nM TNF for various times before fractionation of proteins on a 7% polyacrylamide gel. A Western blot was probed with anti-phospho-Akt (Upper) and then with anti-Akt (Lower).