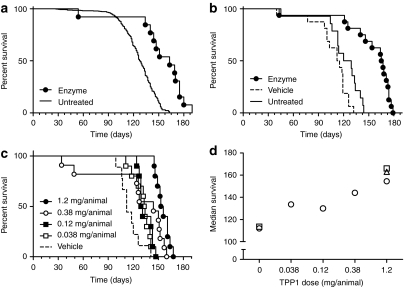
Figure 5.
Effect of intrathecal administration of recombinant human TPP1 (rhTPP1) on survival. Four week-old Tpp1(−/−) animals were either administered three daily infusions of 40 µl rhTPP1 at the indicated concentration, artificial cerebrospinal fluid (CSF) vehicle, or left untreated. (a) Enzyme treated (10.0 µg/µl, total dose = 1.2 mg rhTPP1/animal) (n = 13) compared to historical untreated controls (n = 601). Median survival was 163 and 128 days, respectively (P < 0.0001 by the Mantel–Cox test). (b) Vehicle treated (n = 16), enzyme treated (10.0 µg/µl, total dose = 1.2 mg rhTPP1/animal) (n = 16) and untreated (n = 14). Median survival was as follows: vehicle, 113.5 days; enzyme, 164 days; untreated, 124.5 days. Pairwise results of Mantel–Cox tests: enzyme versus vehicle or enzyme versus untreated, P < 0.0001; vehicle versus untreated, P = 0.0144). (c) Enzyme treated (10.0, 3.16, 1.00, or 0.316 µg/µl yielding total dose indicated on graph) and vehicle treated (n = 9–11 animals/group). All treated groups are significantly different from vehicle control (P < 0.05). (d) Median survival for data plotted in panels a (open triangle), b (open squares) and c (open circles).