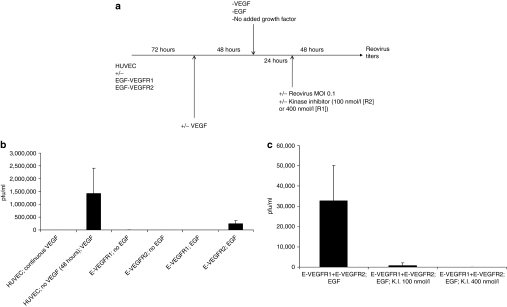
Figure 1.
Signaling through VEGFR2 induces a proviral state for reovirus replication. (a) Human umbilical vein endothelial cell (HUVEC) cells, transduced with either the EGF-VEGFR1 or EGF-VEGFR2 chimeric receptors, as described in Materials and Methods section, were treated as shown. Briefly, 72 hours following transduction, cells were washed and replated in medium containing, or lacking (“VEGF deprivation”), VEGF. Forty-eight hours later, the media were changed again and cells were either kept in the absence of VEGF, or were exposed either to VEGF165 (6 ng/ml) or to EGF (10 ng/ml) (“VEGF/EGF burst”). Twenty-four hours later, cells were exposed to reovirus either in the continued presence, or absence, of VEGF or endothelial growth factor (EGF). Forty-eight hours later, viral titers were determined by plaque assay. (b) The protocol of a above was carried out using (i) HUVEC cells grown continually in VEGF; (ii), HUVEC deprived of VEGF followed by a VEGF burst; (iii), HUVEC/EGF-VEGFR1 grown continually in the absence of VEGF but with no EGF burst,50 HUVEC/EGF-VEGFR2 grown continually in the absence of VEGF but with no EGF burst; (iv), HUVEC/EGF-VEGFR1 grown continually in the absence of VEGF and treated with an EGF burst; (v), HUVEC/EGF-VEGFR2 grown continually in the absence of VEGF and treated with an EGF burst. Titers of reovirus released following each experimental set of conditions, in triplicate wells, are shown. (c) The protocol of a above was repeated using HUVEC cells transduced with both the EGF-VEGFR1 and EGF-VEGFR2 chimeric receptors (at the same levels as used individually in b) and treated with VEGF deprivation followed by EGF burst in the absence of any kinase inhibitor (i); in the presence of kinase inhibitor at 100 nmol/l which selectively inhibits VEGFR2 (ii); or in the presence of kinase inhibitor at 400 nmol/l which inhibits both VEGFR1 and VEGFR2 signaling (iii). Titers of reovirus released following each experimental set of conditions, in triplicate wells, are shown.