Abstract
The first stereospecific synthesis of polyneuridine aldehyde (6), 16-epi-vellosimine (7), (+)-polyneuridine (8), and (+)-macusine A (9) has been accomplished from commercially available D-(+)-tryptophan methyl ester. D-(+)-Tryptophan has served here both as the chiral auxiliary and the starting material for the synthesis of the common intermediate, (+)-vellosimine (13). This alkaloid was available in enantiospecific fashion in seven reaction vessels in 27% overall yield from D-(+)-trytophan methyl ester (14) via a combination of the asymmetric Pictet-Spengler reaction, Dieckmann cyclization, and a stereocontrolled intramolecular enolate-driven palladium-mediated cross-coupling reaction. A new process for this stereocontrolled intramolecular cross-coupling has been developed via a copper-mediated process. The initial results of this investigation indicated that an enolate driven palladium-mediated cross-coupling reaction can be accomplished by a copper-mediated process which is less expensive and much easier to work-up. An enantiospecific total synthesis of (+)-polyneuridine aldehyde (6), which has been proposed as an important biogenetic intermediate in the biosynthesis of quebrachidine (2), was then accomplished in an overall yield of 14.1% in 13 reaction vessels from D-(+)-tryptophan methyl ester (14). Aldehyde 13 was protected as the Na-Boc aldehyde 32 and then converted into the prochiral C (16)-quaternary diol 12 via the practical Tollens’ reaction and deprotection. The DDQ-mediated oxidative cyclization and TFA/Et3SiH reductive cleavage served as protection/deprotection steps to provide a versatile entry into the three alkaloids, polyneuridine aldehyde (6), polyneuridine (8) and macusine A (9) from the quarternary diol 12. The oxidation of the 16-hydroxymethyl group present in the axial position was achieved with the Corey-Kim reagent to provide the desired β-axial aldehydes, polyneuridine aldehyde (6) and 16-epi-vellosimine (7) with 100% diastereoselectivity.
Introduction
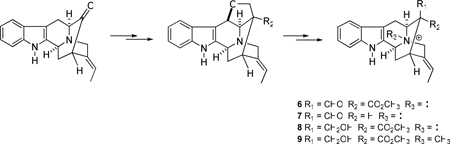
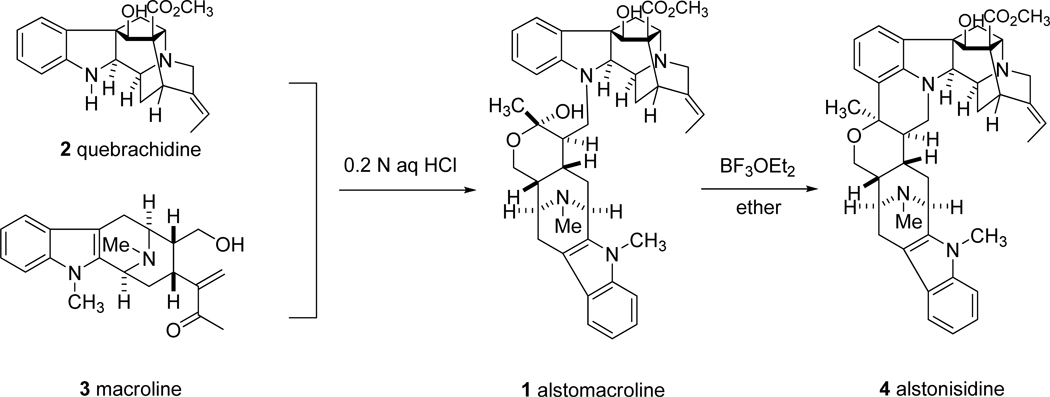
Indole alkaloids have long held a prominent position in the history of natural products chemistry because of their structural similarity to the essential amino acid tryptophan and related metabolites, such as the neurotransmitter serotonin. New alkaloids have been isolated from a variety of sources with increasing frequency and characterized via the latest spectroscopic techniques; moreover, thousands of alkaloids have been obtained from plant sources worldwide.1–6 Bisindole alkaloids are the dimeric forms of these natural products and they have been isolated from various species of plants accompanied by their monomeric progenitors.7 (+)-Alstomacroline (1), a bisindole alkaloid, was isolated from the root bark of A. macrophylla collected in Thailand and exhibited activity against malaria parasites (IC50 of 1.12 µM against the Plasmodia falciparum K1 strain).8,9 A partial synthesis of 1 was reported by Le Quesne et al.10 via a biomimetic coupling process of the two components, (+)-quebrachidine (2) and macroline (3) (Figure 1).
Figure 1.
The partial synthesis of alstomacroline (1).
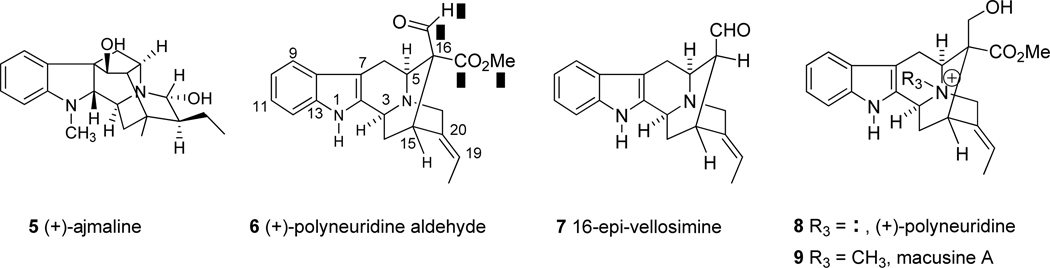
(+)-Ajmaline (5) was isolated from the roots of Rauwolfia serpentina in 1931 by Siddiqui11 It is a clinically important cardiovascular indole alkaloid12–17 with historical significance7 and has been extensively used in Europe to treat arrhythmias. Although (+)-ajmaline (5) itself represents a class of monoterpene alkaloids, it is also related to the sarpagine bases,18–20 which contain one or two functional groups at C-16.
The investigation of the biogenetic link between the sarpagine and ajmaline related alkaloids derives from the isolation of important biogenetic intermediates and specific enzymes from the biosynthesis of ajmaline, which is known as the ajmaline pathway by Stoeckigt.21–24 All the major reactions which have occurred in cell suspension cultures of Rauwolfia have been established at the enzymatic level. Tryptamine and secologanin are converted into polyneuridine aldehyde (6) by the reactions catalyzed by strictosidine synthase (SS), strictosidine synthase (SG) and sarpagine bridge enzyme (SBE), respectively. In addition, the enzyme, (PNAE) has been shown to catalyze the central reaction which transforms polyneuridine aldehyde (6) into 16-epi-vellosimine (7).21–23 This latter alkaloid has been identified as the immediate precursor for the biosynthesis of the ajmaline monoterpenoid alkaloids. Four major special enzymes (vinorine synthase, vinorine hydroxylase, vomilenine reductase, and norajmaline N-methyl-transferase) are involved in this biosynthetic pathway, which eventually provides ajmaline (5) from epi-vellosimine (7).21–24
The unique and complex architecture of the above-mentioned indole alkaloids depicted in Figures 1 and 2, coupled with their largely unexplored potential in medicine or as tools for biological studies, make these compounds attractive targets for total synthesis. (−)-Vincamajinine and its ring–A substituted analogue, (−)-11-methoxy-17-epi-vincamajine, have been recently synthesized by Yu et al.25,26 In addition, an approach to the synthesis of (−)-2 has also been reported by Martin.27 1997, 1997 However, no total synthesis of polyneuridine (8), polyneuridine aldehyde (6) nor of 16-epi-vellosimine (7) (Figure 2) has been reported. Herein, the initial goal was to develop an efficient enantiospecific and stereo-controlled route for these specific ajmaline-related indole alkaloids, which contain a methoxycarbonyl group at C-16. The major challenges to the synthesis of this group of indole alkaloids related to (+)-quebrachidine (2), include generation of the C-16 quaternary carbon center, complete control of the stereochemistry at C-2 and C-17, formation of the C(19)–C(20) olefinic bound in the E configuration and development of an efficient route for the preparation of the rigid hexacyclic system which contains a C-16 carbomethoxy group. Inspired by the biogenetic connection between the sarpagine and ajmaline-related alkaloids proposed earlier,28,29 Stoeckigt has reported a biosynthetic route via polyneuridine aldehyde (6) which contains the unique C-16 axial aldehyde function located in the middle of the ajmaline biosynthetic pathway.30 A related process is the transformation of aldehyde 6 to the previously unknown biogenetic intermediate,21 16-epi-vellosimine (7), and provides a direct precursor for the biosynthesis of the ajmaline skeleton.21–23,30
Figure 2.
Important biogenetic intermediates and synthetic targets.
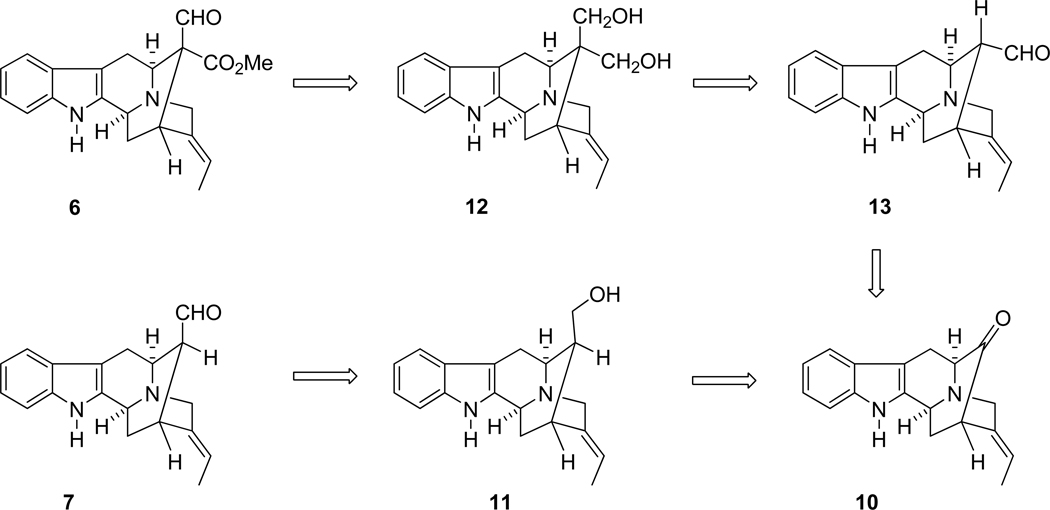
As illustrated in Scheme 1, in a retrosynthetic sense, both polyneuridine aldehyde (6) and 16-epivellosimine (7) might be available via a common intermediate, the E-ethylidene ketone 10. This ketone 10 could then be converted into E-16-epinormacusine B (11),31 which could be employed as an intermediate in the total synthesis of 7. The synthesis of the axial aldehyde function of 6 could be approached by selective oxidation of the Na-H diol 12. The latter intermediate, which contained a quaternary center at C-16 might be obtained from vellosimine (13) via the Tollens reaction in one step. The diol 12 which results might be further oxidized selectively to provide entry into polyneuridine aldehyde 6 in a stereoselective fashion.
SCHEME 1.
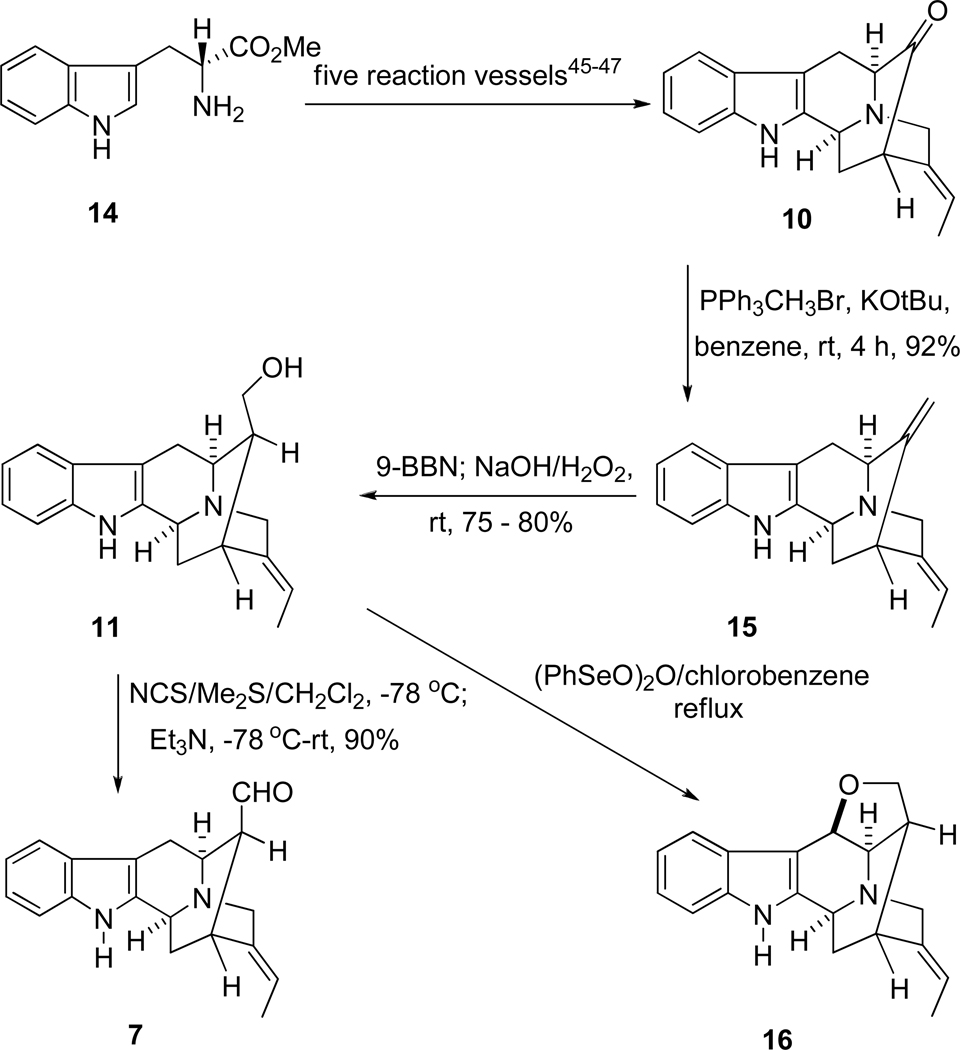
In this regard, the E-ethylidene ketone 1032 had been synthesized in enantiospecific fashion in five steps from D-(+)-tryptophan methyl ester 14 (Scheme 2) via the asymmetric Pictect-Spengler reaction, Dieckmann cyclization, and palladium mediated cross coupling previously.32–34 In addition, two other palladium-mediated enolate driven cross-coupling processes have been employed to provide ketone 10, as well as a copper-mediated method to generate related ketones (See below). This synthetic strategy was an efficient way to provide pentacyclic ketone 10 on gram scale. With the advantage of this method, attention turned to the synthesis of the Na-H axial aldehyde at C-16 in order to construct the two desired aldehydes 6 and 7 in an enantiospecific fashion.
SCHEME 2.
Results and Discussion
Synthesis of 16-epi-vellosimine (6) and strategy for the construction of the axial aldehyde at C-16
The synthesis of 16-epi-vellosimine (6) began with the pentacyclic ketone 10,35–37 as mentioned. The E-16-epinormacusine B (11) was obtained from 10 via a Wittig reaction coupled with a hydroboration/oxidation following the published procedure.31 When the two double bonds in the diene 15 were compared, it was felt the less hindered double bond at C(16)–C(17), relative to the ethylidene at C(19)–C(20), would be more readily attacked by the hindered hydroborating reagent. This was in agreement with the earlier work of Magnus.38,39 Treatment of diene 15 with 9-BBN was followed by oxidative workup to provide the alcohol 11 as the only detectable diastereomer in 80% yield (Scheme 2). When the work up was altered to the procedure (NaBO3) of Kabalka, the yield was 90%.
Once the β-axial alcohol 11 was in hand, the axial aldehyde 7 could, presumably, be obtained via selective oxidation under mild conditions, if epimerization to the thermodynamically more stable alpha equatorial aldehyde could be avoided. Recently, during the total synthesis of ajmaline40 and the vincamajinine-related alkaloids25 entry into the 16-Na-methyl axial aldehyde was achieved,25 as well as evidence for the close synthetic relationship to the indolenine group. In the previous approach to the related Na-methyl diol,25 the TPAP selective oxidation of the β-axial alcohol on the Boc protected 16-functionalized quaternary diol as well as the Na-methyl diol was employed to provide a 16-β-axial aldehyde (dr>8:1);26,41 however, the synthesis of aldehydes in the Na-H series was more difficult. This was, presumably, due to the acidic nature of the indole Na-H function, as well as the lability of the Na-H 2,3-indole system in the presence of oxidative reagents. Moreover, it was well known the aldehyde function at C-16 preferred the α-equatorial stereochemistry.42 The axial aldehyde function of 7 could be easily epimerized into the sarpagine series of alkaloids,30 even on silica gel chromatography. Previously, the difficulty in isolation and preparation of Na-H axial aldehydes at C-16 had prevented use of this biogenetic-type strategy for the synthesis of ajmaline/quebrachidine-like alkaloids. Consequently, the strategy here rested on the use of the proper mild oxidative reagents to provide the desired 16-epivellosimine (7) without epimerization at C-16. Moreover, polyneuridine aldehyde could be synthesized by extension of this approach to the C-16-quaternary Na-H diol 12.
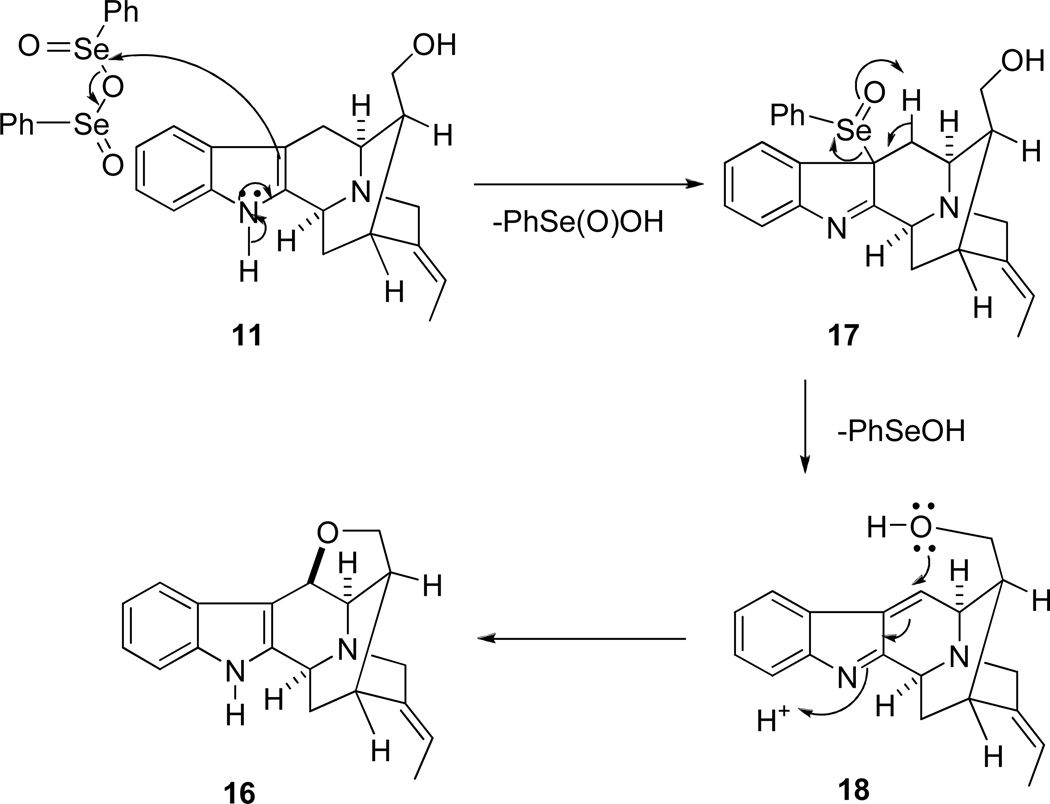
Various oxidative conditions were then attempted to convert monol 11 into aldehyde 7 to furnish the less stable axial aldehyde. These previous efforts (Swern oxidation,43 Dess-Martin periodinate44 and IBX45) resulted in the decomposition of the starting material or the isolation of the equatorial aldehyde. Analogous to the previous work of Sakai et al.,46 it was found the Corey-Kim reagent47 reacted readily with the alcohol 11 in the presence of Et3N at −78 °C to give 16-epi-vellosimine (7) in high yield, when this was allowed to warm to room temperature. The spectroscopic properties of synthetic 7 were similar to the pattern reported for (+)-vellosimine;48 however, examination of the proton NMR spectrum indicated the aldehydic peak had shifted from δ 9.65 to 9.16 (Table I, SI). This was the typical chemical shift reported for the hindered axial aldehyde.49 Herein, (+)-16-epi-vellosimine (7) had been prepared in a short synthetic sequence from D-(+)-tryptophan methyl ester (14) in nine reaction vessels in 23% overall yield. This material was epimerized into (+)-vellosimine on stirring with base or exposure to silica gel during chromatography, for comparison proposes to natural (+)-vellosimine. In addition, it was noteworthy that the hydroxymethyl group of 11 was converted into the ether 16 by oxidative cyclization when it was treated with benzeneselenic anhydride.50 The ether, dehydro-16-epinormacusine B (16), had been previously prepared by DDQ-mediated oxidative cyclization.31 A potential mechanism of oxidation of indole 11 to provide ether 16 is illustrated in Scheme 3.
SCHEME 3.
The Corey-Kim oxidation can be employed for a wide variety of primary and secondary alcohols with the exception of allylic and benzylic alcohols. The polar solvent CH2Cl2 was chosen in this approach. In this oxidation a side-reaction may occur in which the alcohol formed the corresponding methylthiomethyl ether (ROCH2SCH3) by deprotonation. This side product was observed in the case of the preparation of polyneuridine aldehyde (6); however, in the analogous approach to 16-epi-vellosimine (7), a clean and pure aldehyde was obtained in 90% yield. The reaction conditions for the Corey-Kim oxidation are mild and tolerate most functional and protecting groups. Therefore, it was employed in the next sequence for the total synthesis of polyneuridine related alkaloids.
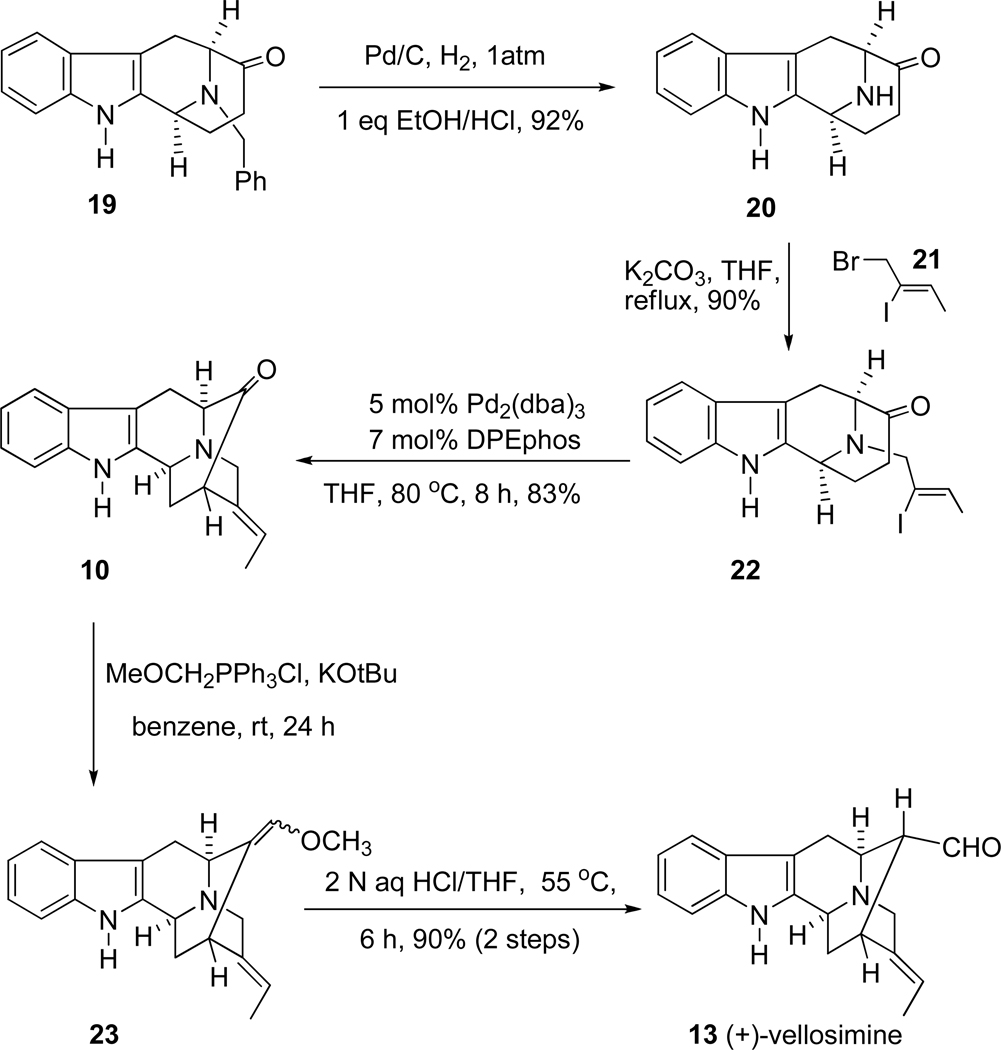
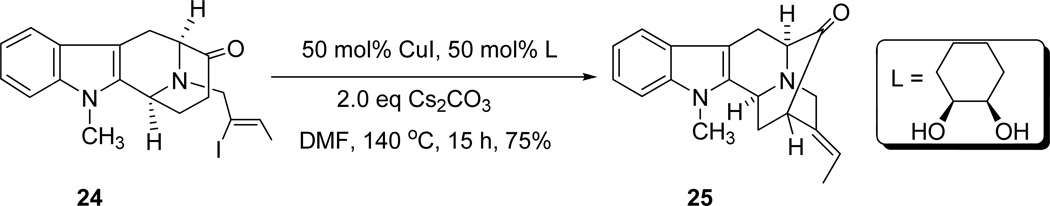
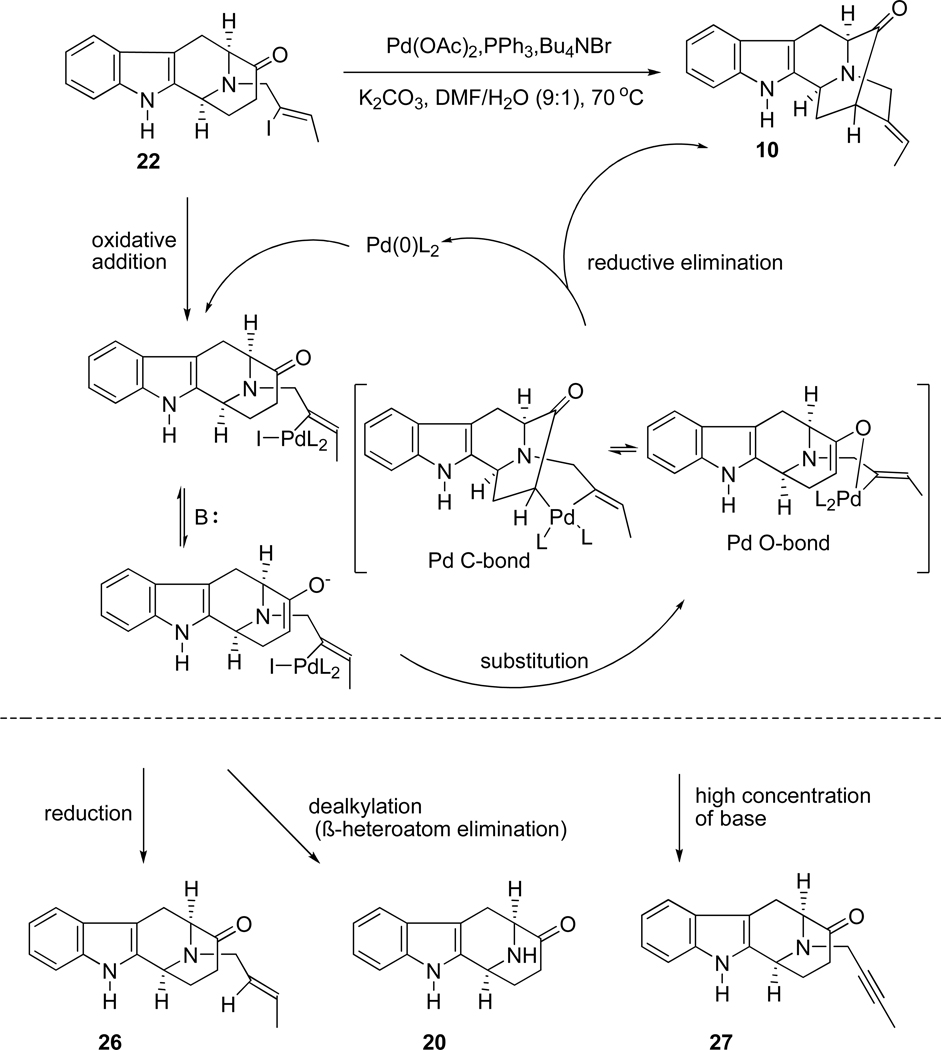
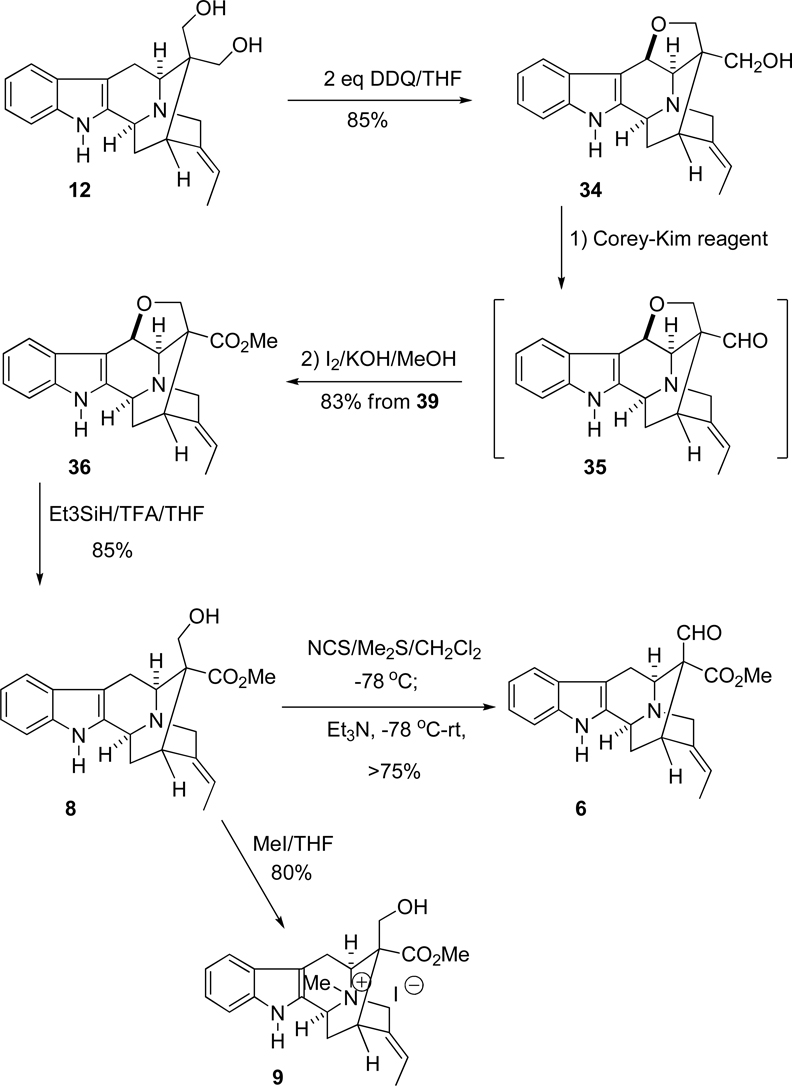
The key intermediate, (+)-vellosimine (13) was prepared from the common intermediate, E-ethylidene ketone 10, which was synthesized via the asymmetric Pictect-Spengler reaction, Dieckmann cyclization, and palladium mediated cross coupling in high overall yield.35–37 As illustrated in Scheme 4, the route began with the readily available (−)-Na-H tetracyclic ketone 19, which was subjected to the conditions of catalytic hydrogenolysis to remove the benzyl function in 92% yield. This base 20 was reacted with the (Z)-1-bromo-2-iodo-2-butene 21 in the presence of K2CO3 in THF at reflux to provide the Nb-alkylated ketone 22 in 90% yield. The ketone 22 was initially converted into the desired ketone 10 in 80% yield via an intramolecular palladium (enolate mediated) cross-coupling reaction (See SI for experimental details)51,52 analogous to the process developed by Wang et al.32,53,54 Further development of this palladium-mediated cross coupling reaction has been achieved when ketone 22 was stirred in combination with 5.0 mol% Pd2(dba)3, 7.0 mol% DPEphos and 1.5 equivalents of NaOtBu in THF at 80 °C for 8 hours. The desired ketone 10 was obtained in 83% yield which was superior to the previously reported results (Scheme 5). In addition, another process was also carried out with ketone 22 in combination with 6.0 mol% Pd(PPh3)4 and a base PhOK52 (which was generated previously in the reaction vessel from 2.0 equivalent of PhOH and 1.5 equivalent of KOtBu). The desired ketone 10 was obtained in 80% yield which was the same as reported in previous processes [Improvements and previous results are summarized in Table 1 (SI)], but less of the vinyl acetylene byproduct was observed. Ketone 10 was then subjected to the Wittig reaction with methoxymethyl triphenylphosphonium chloride and anhydrous potassium tert-butoxide to provide a mixture of two stereoisomeric enol ethers represented by olefin 23. After a short wash column, the mixture of enol ethers 23 was hydrolyzed under acidic conditions to provide vellosimine 13 in 90% yield (overall yield for two steps). Since the aldehyde at C(16) obtained from 23 preferred the more stable α configuration because of a syn pentane interaction with the indole methylene bridge, the mixture was simply stirred until all of the β-epimer was converted into the more stable, natural α epimer present in (+)-vellosimine 13. Because of the cost of palladium an alternative, viable and cheaper route was investigated in regard to the cross-coupling to provide ketone 10. The recent report55 on the copper-mediated preparation of vinyl sulfides encouraged use of the same catalytic system (CuI in combination with 1,2-cis-cyclohexanediol) for this cross-coupling reaction to obtain ketone 10 from vinyl iodide 22. The treatment of 24 in combination with 50 mol% of CuI and 50 mol% of 1,2-cis-cyclohexanediol as a ligand and 2.0 equivalents of Cs2CO3 as a base in DMF at 140 °C for 15 hours provided pentacyclic ketone 25 in 75% yield (Scheme 5). This was the best yield in this copper-mediated strategy obtained to date and it was much easier to work-up this reaction than the corresponding palladium mediated process (Scheme 5). A series of ligands in combination with CuI were screened and optimized (See SI, Scheme 2); however, this process has not worked as well in the Na-H series of interest here (See SI for details). Further work is underway to maximize the yields in both the Na-methyl case 25 and the Na-H case 10.
SCHEME 4.
SCHEME 5.
A plausible Buchwald-Hartwig catalytic cycle for the enolate-driven palladium catalyzed α-vinylation reaction is illustrated in Scheme 6. It was felt the active Pd(0)L2 species was generated primarily by reduction of Pd(OAc)2 with triphenylphosphine in this phosphine-assisted catalytic cycle.56,57 The active Pd(0)L2 species could then undergo oxidative addition on the vinyl iodide to form a vinylpalladium(II) iodide complex. Ligation to the oxygen and rearrangement to the carbon atom at C(15) can then occur. Finally, reductive elimination of the carbon bound Pd(II) intermediate would furnish the desired E-olefin 10 while regenerating the active Pd(0)L2 species. A key to the success of this Pd-mediated cross coupling reaction rested on the enolate concentration. This must be high enough to permit ligand substitution to occur.36 It was well known that vinyl iodides would be quite reactive in the oxidative-addition step. If nucleophilic substitution of the iodide by the nucleophilic enolate in the coupling process was comparably slow, the rate of byproduct formation would be significant. Eventually, a highly reproducible yield could be obtained by employing an excess of the phosphine ligand.32 In the case of different substrates, sometimes it was necessary to carry out the reaction with a 10:1 ratio of PPh3 (30 mol%) to Pd(OAc)2 (3 mol%). The process then took three days to go to completion in comparison to that of five hours required under the original conditions, but the amount of byproducts (Scheme 6) was decreased. The yield of the sequence in this series was 81%. Furthermore, the best solvent system to date was DMF/H2O (9/1), alternation to CH3CN, pure DMF, or DMF/H2O (4/1) resulted in no reaction or low yields.58 Because the conditions employed for the enolate driven palladium coupling process were different from the recent Buchwald-Hartwig arylation reactions,32 efforts were directed toward intramolecular α-vinylation under the conditions of the arylation process.32 This might permit lower catalyst loading32,51 and be effective for both E and Z ethylidenes. The homogenous reaction conditions might permit a faster rate and thereby facilitate scaleup. In fact, Liao first reported a Pd(0) catalyzed α-vinylation process in which the cheaper DPEphos was employed in the case of a Z-ethylidene59 for the synthesis of (−)-koumidine.60 Moreover, utilization of this ligand for the synthesis of the E-ethylidene 10 was also executed by Liao et al. in 80% yield.59 However, a related approach has also been effective based on a palladium-catalyzed coupling of amino-tethered vinyl halides with ketones reported by Solé, Bonjoch et al.51,52 In this regard, ketone 22 was heated to 70–75 °C in the presence of a palladium (0) triphenylphosphine catalyst and potassium phenoxide. The intramolecular cyclization took place to afford ketone 10 in 80% yield, again in stereospecific fashion (See SI, Scheme 1). The use of the weaker potassium phenolate base limited the presence of any acetylene byproduct formed from loss of HI from halide 22.
SCHEME 6.
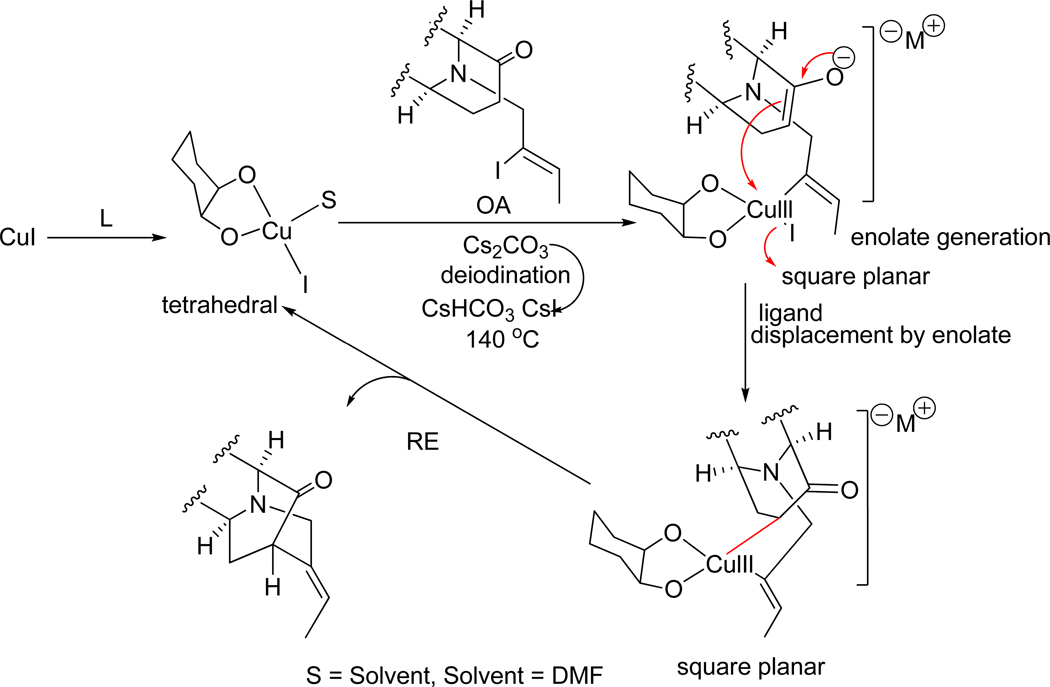
In the case of the copper-mediated process for the above mentioned cross-coupling (Scheme 5), a working mechanistic hypothesis is illustrated in Scheme 7. It is believed that 1,2-cis-cyclohexanediol ligated with CuI and solvent in a tetrahedral fashion. The deiodination of the iodide ligand by a base and a subsequent oxidative addition to vinyl iodide 24 with copper may lead to Cu(III) with a negative charge on the overall complex. Enolate generation and subsequent ligand displacement by the enolate would furnish the square planar complex (Scheme 7). It is believed that the final step involved reductive elimination to provide the desired ketone 25 in the usual fashion. It is important to point out that neither 1,2-trans-cyclohexanediol (See SI) nor ethylene glycol worked as well as the 1,2-cis-cyclohexanediol in this procedure.
SCHEME 7.
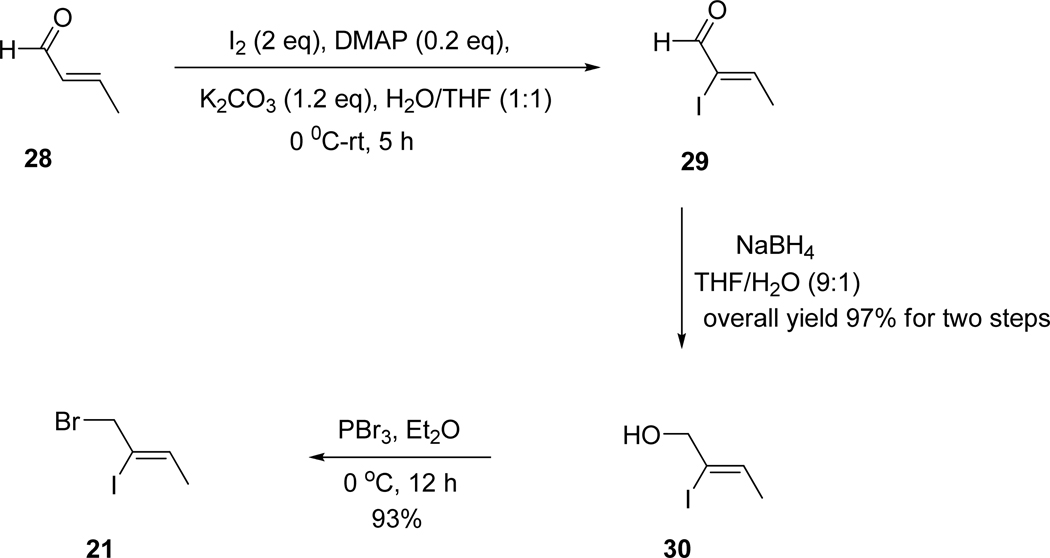
Initially, the vinyl iodide 21 required for this route was synthesized according to a literature procedure.61 However, the free-radical hydrostannation of the propargyl alcohol was difficult and the desired iodide was extremely hard to purify. Importantly, as illustrated in Scheme 8, an improved method was recently developed62 to prepare 21 via α-iodination of an α,β-unsaturated aldehyde 28 under the conditions of Kraft63 which provided only the Z-isomer of 29. After work-up without further purification of 29, the subsequent reduction61 of 29 provided the desired alcohol 30 in 97% overall yield. This alcohol 30 was treated with PBr3 in dry ether at 0 °C for 12 hours to provide the desired bromide 21 in 93% yield (See Experimental Section for details). The sequence was scaled up to the 200 g level scale starting from crotonaldehyde 28. Overall, vellosimine 13 was synthesized in gram quantities; moreover, some optimized reaction conditions have been updated in this report to provide the best synthesis of 13, to date.
SCHEME 8.
With gram quantities of vellosimine 13 in hand, attention turned to the enantiospecific preparation of the two biogenetic intermediates polyneuridine (8) and polyneuridine aldehyde (6). This route began with the Na-H diol 12 (Scheme 1) and could, presumably, be employed for the synthesis of quebrachidine (2). Instead of the early-stage TPAP-mediated regioselective oxidation of the C-16 axial hydroxymethyl function employed by Yu,25 in the total synthesis of vincamajinine, it was felt selective protection of the axial hydroxymethyl group in diol 12 would differentiate between the two hydroxymethyl groups to provide polyneuridine (8). Analogous to the synthesis of 16-epi-vellosimine (7) developed here, the Corey-Kim oxidation would be employed to alter the oxidation state of the C-16 axial hydroxymethyl group in ester 8 to provide polyneuridine aldehyde (6).
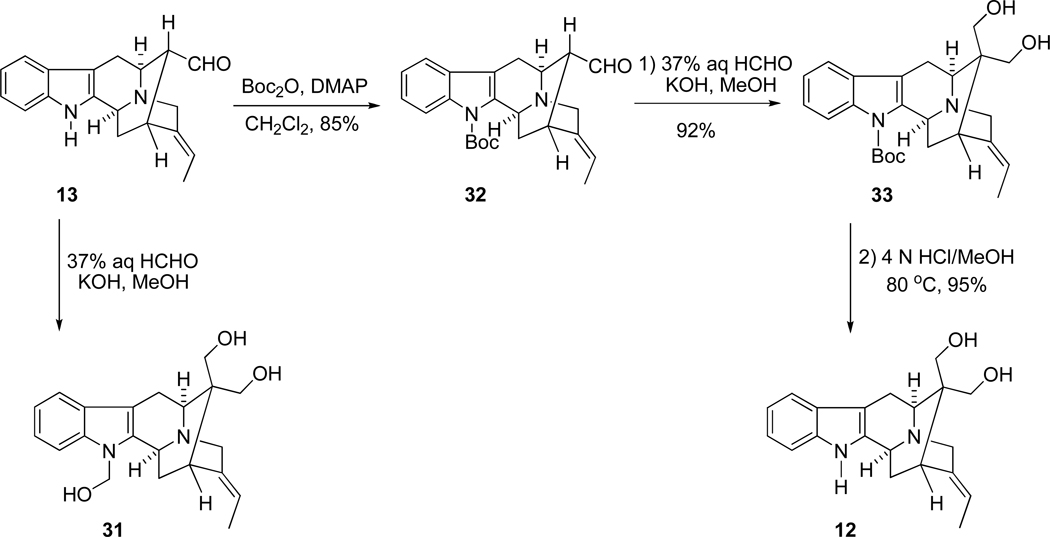
In order to construct the quaternary carbon center at C-16, numerous efforts (aldolizations, alkylations, and acylations) were originally carried out, but they were not successful.64–67 Gratifyingly, it was found that the aldehydic group at C-16 in Na-methylvellosimine could be converted into its related diol after 60 hours in 88% yield via the Tollens reaction by using 37% aqueous formaldehyde (5 equivalents) and KOH (10 equivalents) in methanol.26 However, this was not as easy in the Na-H system of vellosimine (13). As illustrated in Scheme 9, the undesired triol 31 formed in 83% yield when 13 was treated with formaldehyde and base. Attempts to readily remove the Na-H substituted hydroxymethyl function were not successful. Therefore, it was necessary to prohibit reaction of formaldehyde with the indole Na-H moiety of vellosimine (13). Consequently, aldehyde 13 was protected as the Na-Boc intermediate with Boc2O in the presence of DMAP to afford 32 in 85% yield. It was then converted into the Boc protected prochiral C-16-quaternary diol 33 via the Tollens-like reaction (crossed Cannizzaro process), and this was followed by deprotection under acidic conditions. The prochiral quaternary carbon center at C-16 that contained the structurally hindered diol of 12 was constructed in two steps within one reaction vessel in this process. More importantly, because of the symmetry of the two diol moieties at C-16, generation of a new chiral center was avoided. However, the two hydroxymethyl groups are prochiral which was useful, as planned.
SCHEME 9.
With the success in the synthesis of the diol 12, selective protection of the C-16 axial hydroxymethyl group in preference to the equatorial hydroxymethyl group was achieved with dichlorodicyanobenzoquinone (DDQ)-mediated oxidation. As illustrated in Scheme 10, following a process developed earlier in this laboratory,68 the diol 12 was treated with DDQ in anhydrous THF to give the ether 34, which permitted further modification of the equatorial hydroxymethyl group. The hydroxymethyl group which remained was converted into the methyl ester 35 via the 2 steps detailed below. Various oxidative reagents (i.e., TPAP,69 Dess-Martin periodinate,44 and benzeneselenic anhydride50) were employed to furnish the equatorial aldehyde 36; however, most of these efforts resulted in the decomposition of the starting material or cleavage of the C(6)–C(17)-oxygen bridge. Again, the conditions of the Corey-Kim oxidation could be employed successfully in this system; the monol 34 was converted into the equatorial aldehyde 35 in 90% yield. The aldehyde function of intermediate 35 was then oxidized to the methyl ester 36 with I2, and KOH in MeOH, following the work of Yamamoto et al.33,68,70 This illustrated atom economy at its best. After oxidative formation of the α-methyl ester 36 at C-16, the ether bond was reductively cleaved with TFA/Et3SiH68 in 85% yield to provide polyneuridine (8) [FTIR 3267 cm−1(OH), 1736 cm−1(CO2Me)] (Figure 1, S.I.). As illustrated in Table II (SI), the spectral data of polyneuridine were in excellent agreement with the natural product.71,72 Furthermore, the important biogenetic intermediate, polyneuridine aldehyde (6) [FTIR 1731cm−1(CO2Me), 1707cm−1(CHO)] was then obtained by a second Corey-Kim oxidation (Figure 2, SI). Consequently, aldehyde 6 could be prepared from D-(+)-tryptophan methyl ester (14) in 13 reaction vessels in 14.1% overall yield. Reduction of polyneuridine aldehyde 6 with sodium borohydride returned polyneuridine 8, which confirmed the presence of the aldehyde moiety in 6. Finally, quarternization of the Nb nitrogen function in polyneuridine (8) with MeI provided the Nb-methiodide salt, macusine A (9) in 80% yield. This was the first total synthesis of these three alkaloids and was accomplished in stereospecific fashion.
SCHEME 10.
It is important to note the Corey-Kim oxidation provided an extremely mild method to complete the key oxidation of the C-16-hydroxymethyl group of the indole alkaloids, 16-epi-vellosimine (7) and polyneuridine aldehyde (6) in the Na-H series in the absence of epimerization to the more stable C-16 equatorial aldehyde as in 13. Treatment of alcohols 11 and 8 with 5 equivalents of the Corey-Kim reagent in CH2Cl2 at −78 °C for 2 hours was followed by the standard work-up46 to provide the respective aldehydes in high yield. The DDQ oxidation of diol 12 to provide ether 34 was not simple. The oxidation was run at a lower temperature in a dry ice bath under argon and approximately 50% of the desired ether 34 appeared on TLC in 5 minutes. This was after the addition of 1 equivalent of DDQ. Since no further change occurred after one half an hour, another equivalent of DDQ was added to the mixture and no obvious change was observed over a two hour period at this temperature. The reaction mixture was allowed to warm to rt at which time the process went to completion. Analysis by TLC indicated that only one component (Scheme 10) was present under these conditions, and that was ether 34.
Conclusion
The first stereospecific total synthesis of 16-epi-vellosimine (7), (+)-polyneuridine (8), (+)-polyneuridine aldehyde (6), and macusine A (9) has been accomplished from commercially available D-(+)-tryptophan methyl ester (14). The asymmetric Pictet-Spengler reaction and palladium-mediated enolate cross coupling process were key steps to construct the sapargine skeleton. An alternative palladium-mediated enolate cross-coupling was developed (Scheme 4 and See Experimental Section) and the use of a milder base PhOK generated in-situ may minimize the formation of the acetylene byproduct (See SI, Scheme 1). In addition, the application of the copper-mediated cross-coupling process in the case of the Na-Me iodo ketone has been realized with comparable results. The Pd to Cu switch has permitted extension of this process to one that is cheaper and much easier to work-up. A stereocontrolled formation of the C-16 quaternery carbon center was accomplished by a very practical Tollens’ (crossed Cannizzaro) reaction. This important transformation established the prochiral hydroxymethyl groups at C-16 without the need for chiral reagents or asymmetric induction. Presumably, this robust reaction could be scaled up to kilogram scale in this series. The DDQ-mediated oxidative cyclization and TFA/Et3SiH reductive cleavage served as protection/deprotection steps in order to provide a versatile entry into these alkaloids. The chemospecific and regiospecific oxidations with the Corey-Kim reagent were key steps in these syntheses to provide the desired aldehydes. This constituted the first stereospecific synthetic solutions to the axial aldehydes in 16-epi-vellosimine (7) and polyneuridine aldehyde (6) and should also provide access to the ajmaline/quebrachidine alkaloids.
Experimental
Conversion of Na-H-17-Hydroxysarpagan-16-methanol (12) into Na-H-17-Hydroxy-sarpagan-16-methyl-6-cyclic ether (34)
The recrystallized (from CH2Cl2) DDQ (253 mg, 1.111 mmol) was added to a solution of diol 12 (180 mg, 0.556 mmol) in dry THF (10 mL) at −78 °C (dry-ice bath). The black-blue colored mixture which resulted was stirred at this temperature and gradually allowed to warm to rt. The reaction was completed in 20 min to 0.5 h until analysis by TLC indicated the absence of starting material. The mixture was then diluted with CH2Cl2 (90 mL), washed with a saturated solution of aq NaHCO3 (10 mL × 2) and then concentrated under reduced pressure. The residue was again dissolved in a mixture of CH2Cl2 (90 mL) and MeOH (10 mL), after which it was washed with a solution of 20 % aq NH4OH (30 mL × 2) and brine (2 × 10 mL). It was dried over K2CO3. The organic layer was then removed under reduced pressure, and the residue which resulted was flash chromatographed (silica gel, CH2Cl2/MeOH = 20:1) to provide the cyclic ether 34 (161 mg, 90%): FTIR (NaCl) 3745, 1698, 1450, 744, 464 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 1.61 (d, J = 4.5 Hz, 3H), 1.78 (m, 1H), 1.90 (m, 1H), 2.80 (d, J = 6 Hz, 1H), 2.95 (s, 1H), 3.15 (m, 1H), 3.22 (m, 1H), 3.40 (s, 1H), 3.58 (d, J = 10.5 Hz, 1H), 3.68 (d, J = 3 Hz, 1H), 3.92 (dd, 1H), 4.68 (t, 1H), 5.34 (m, 1H), 5.42 (d, J = 3 Hz, 1H), 6.98 (t, 1H), 7.04 (t, 1H), 7.31 (d, J = 5.1 Hz, 1H) 7.46 (d, J = 6 Hz, 1H), 10.98 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ 13.6, 29.1, 30.2, 32.0, 46.4, 48.8, 55.4, 63.6, 66.2, 66.7, 103.5, 112.1, 115.3, 118.9, 119.7, 121.4, 127.0, 136.5, 138.6, 144.2. EIMS (70eV) (m/e relative intensity): 322(M+, 100), 305(26), 291(16), 182(41), 169(85). HRMS calcd for C20H22N2O2 322.1681, found 322.1670. This material was used directly in the next step.
Alternative Pd-Catalyzed Cyclization of (6S, 10S)-(−)-9-oxo-12-[(Z)-2´-iodo-2´-butenyl]-6,7,8,9, 10,11-hexaydro-6,10-imino-5H-cyclooct[b]indole (22) to Provide Pentacyclic ketone (10)
The Nb-Z-2´-iodo-butenyltetracyclic ketone 22 (1 g, 2.46 mmol) was dissolved in anhydrous THF (60mL). To this solution, NaOtBu (0.354 g, 3.69 mmol), DPEphos (0.0927 g, 7.0 mol %) and Pd2(dba)3 (0.112g, 5.0 mol %) were added. This reaction mixture was degassed under argon and placed in a preheated oil bath at 80 °C for 5 hrs. The reaction solution was the quenched with water and extracted with EtoAc (3 × 250mL). The organic layer was separated and dried (Na2SO4). The EtOAc was then removed under reduced pressure and residue was flash chromatographed with CH2Cl2/MeOH (4.5:0.5) to provide the coupling product 10 (0.569g, 83% yield). 1H NMR (300 MHz, CDCl3) δ 1.63 (3H, d, J= 6.86 Hz), 2.15–2.20 (1H, m), 2.38 (1H, t, J = 9.87 Hz), 2.97 (1H, dd, J =15.58, 6.20 Hz), 3.27 (1H, d, J = 14.43 Hz), 3.37 (1H, bs), 3.59 (1H, d, J = 5.70 Hz), 3.78 (2H, bs), 4.26 (1H, d, J = 9.11 Hz), 5.49 (1H, q, J = 6.85 Hz), 7.05–7.15 (2H, m), 7.25 (1H, d, J =7.25 Hz), 7.47 (1H, d, J = 7.29 Hz), 7.92 (1H, bs); 13CNMR (75.5 MHz, CDCl3) δ 12.68, 22.39, 36.40, 44.55, 50.83, 55.24, 64.17,105.62, 110.89, 118.54, 119.69, 121.26, 122.00, 126.88, 131.88, 135.87, 136.34, 217.00; CIMS (m/e, relative intensity) 291 (M+ + 1, 100); EIMS (m/e, relative intensity) 278 (M+, 10), 250 (75), 249 (85), 182 (6), 169 (100), 168 (5); HRMS (m/e, relative intensity) required for C18H18N2O 278.1419, found 278.1437. The spectral data of this ketone were identical to the published values.73
Copper-Mediated Cyclization of (6S, 10S)-5-Methyl-(−)-9-oxo-12-[(Z)-2´-iodo-2´-butenyl]-6,7,8,9, 10,11-hexaydro-6,10-iminocyclooct[b]indole (24) to Provide 3-Ethylidine-12-methyl-1,3,4,7, 12,12b-hexahydro-13-hydroxymethyl-2H,6H-2,6-methano-indolo [2,3-α)-quinolizin-13-one (25)
A mixture of tetracyclic ketone 24 (1.00 mmol), CuI (99.99%) (50 mol%), 1,2-cis-cyclohexanediol (50 mol%), and Cs2CO3 (2.0 mmol) was placed in dry DMF (Aldrich sure seal bottle), after which it was degassed (3 times)under reduced pressure at rt and refilled with argon (3 times). The reaction mixture was then placed on a pre-heated oil bath (140 °C) under argon and allowed to stired at 140 °C for 12h. At this point analysis by TLC (silica gel, EtOAc/hexane = 1:1, double runs) indicated the absence of starting material 24. The mixture was cooled to rt, diluted with EtOAc and filtered through celite. The reaction mixture was then washed with H2O (5 × 100 mL), brine (100 mL) and then dried (Na2SO4). The solvent was removed under reduced pressure and the oil which resulted was purified by chromatography on silica gel (EtOAc/hexane: 1:1) to provide pure pentacyclic ketone 25. 1H NMR (300 MHz, CDCl3) δ 1.28 (1H, t, J = 6.9 Hz), 1.85 (3H, s), 2.40 (1H, m), 2.74 (1H, d, J = 12.2 Hz), 2.95 (1H, m), 3.04 (1H,m), 3.30 (1H, m), 3.61 (3H, s), 3.92(1H, d, J = 5.4 Hz), 4.01 (1H, m), 4.43 (1H, d, J = 9.4 Hz), 5.40 (1H, q, J = 6.9 Hz), 7.11 (1H, t, J = 7.5 Hz), 7.22 (1H, t, J = 6.9 Hz) 7.28 (1H, d, J = 8.1 Hz), 7.51 (1H, d, J = 7.5 Hz); 13C NMR (75.7 MHz, CDCl3) δ 12.7, 22.3, 29.3, 35.5, 44.1, 49.7, 55.4, 64.1, 104.4, 108.8, 118.5, 119.1, 119.5, 121.5, 126.4, 132.1, 136.9, 137.4, 215.8. The spectral data for this material were identical to the published values.32,59
Preparation of 1-Bromo-2-iodo-but-2-ene 21 from But-2-enal 28
Potassium carbonate (24 g, 172 mmol), I2 (72.42 g, 286 mmol) and DMAP (3.48 g, 28.6 mmol) successively were added to a solution of crotonaldehyde 28 (10 g, 143 mmol) in a mixture of THF (350 mL) and water (350 mL). After stirring for 4–5 h, the reaction mixture was diluted with EtOAc and washed with a solution of saturated aq Na2S2O3. The organic layer was dried (Na2SO4) and the crude product 29 obtained after evaporation in vacuo was used in the next step without purification. The crude product 29 (143 mmol) was taken up in THF-H2O (9:1) and cooled to 0 °C. The NaBH4 (2.69 g, 71.5 mmol) was added slowly and the reaction was stirred for 1 h. The reaction mixture was quenched with water and extracted with EtOAc (3 × 500mL). The organic layer was concentrated and the residue purified by flash chromatography with silica gel in ethyl acetate: hexane (1:9) to obtain iodide 30 (27.40 g, 97% yield). 1H NMR: δ 5.98 (q, J = 6.3 Hz, 1H); 4.23 (s, 2H); 1.79 (d, J = 6.3 Hz, 3H). The spectral properties of this iodide were identical to the published values.61
(Z)-2-Bromo-2-buten-1-ol 30 (2 g, 10 mmol) was dissolved in anhydrous ethyl ether (20 mL). Phosphorus tribromide (0.380 mL, 4 mmol) was added dropwise to this solution at 0 °C. This reaction mixture which resulted was stirred for 12 h at rt. The reaction was quenched with a cold aq solution of K2CO3 and extracted with ethyl ether after which it was washed with brine. The organic layer was dried (Na2SO4) and concentrated in vacuuo to give 21 (2.45 g, 93% yield) which was directly used in the next step.74 1H NMR: δ 6.08 (q, J = 6.4 Hz, 1H); 4.36 (s, 2H); 1.81 (d, J = 6.4 Hz, 3H). The spectral properties of this bromide were identical to the published values.75
Preparation of Aldehyde 35
To a stirred solution of N-chlorosuccinimide (400 mg, 3.0 mmol) in dry CH2Cl2 (15 mL) was added dimethyl sulfide (1.1 mL, 15 mmol) at 0 °C under argon. A white precipitate appeared immediately after addition of the sulfide. The mixture was cooled to −78 °C (EtOAc-dry ice bath), and stirring was continued for 1 h at −78 °C. The mixture of ether 34 (194 mg, 0.6 mmol) in dry CH2Cl2 (3 mL) was then added into the resulted white complex at −78 °C and the stirring was continued for 2 h at −78 °C. A solution of triethylamine (1.4 mL, 10 mmol) in CH2Cl2 (0.5 mL) was added to the mixture. The stirring was continued for an additional 1 h. The cooling bath was then removed, and after 10 min, ether (20 mL) was added. The organic layer was diluted with a mixture of CH2Cl2 (45 mL) and MeOH (5 mL), after which it was washed with a solution of 1% aq hydrochloric acid (5 mL) and twice with water (2 × 15 mL). The organic layer was separated and dried (Na2SO4). The solvent was removed under reduced pressure to provide the crude aldehyde 35 (180 mg, 93%). Analysis of the 1H-NMR spectrum indicated the presence of the desired aldehydic peak at δ 9.56 or occasionally at 9.60. This material was employed in a later step without further purification.
16-Epi-vellosimine
(7) was prepared from the alcohol 11 ((E) 16-epinormacusine B) following the analogous procedure employed for preparation of 35 above. 7: 1H NMR (300 MHz, DMSO-d6) δ 1.65 (d, J = 8.4 Hz, 3H), 1.75 (t, 1H), 2.02 (m, 1H), 2.26–2.32 (bs, 1H), 2.55–2.66 (m, 1H), 2.52–2.84 (m, 1H), 2.75 (bs, 1H), 3.51–3.69 (m, 2H), 3.76 (dt, 1H), 4.15 (d, J = 9Hz, 1H), 5.30 (q, 1H), 7.106–7.14 (m, 2H), 7.24 (d, J = 6Hz, 1H) 7.43 (d, J = 6 Hz, 1H), 8.18 (s, 1H), 9.16 (s, 1H); 13C NMR (75.7 MHz, CDCl3) δ 12.7, 23.8, 25.1, 26.5, 49.9, 50.1, 53.0, 55.8, 104.7, 110.9, 114.9, 118.0, 119.5, 121.8, 126.2, 136.6, 137.3, 138.2, 200.7. The spectra data were similar to those of the previous synthetic vellosimine 27, except the aldehydic peak had shifted to 9.16 from 9.56 (Table 2, SI).
Oxidation of the Aldehyde 35 into Methyl Ester 36
The crude aldehyde 35 (87 mg, 0.272 mmol) which resulted from the Corey-Kim oxidation was triturated with hexane (6 × 5 mL) to remove impurities. The residue was dissolved in anhydrous MeOH (2 mL), and a solution of 85% KOH (148 mg, 2.21 mmol) and iodine (280 mg, 1.1 mmol) in anhydrous MeOH (4 mL) were successively added at 0 °C. After 6 h, the reaction mixture was diluted with CH2Cl2 (80 mL), washed with a 10% aq solution of Na2S2O3 (20 mL), water (20 mL), brine (20 mL), and dried (Na2SO4). The solvent was removed under reduced pressure, and the residue which resulted was purified by flash chromatography on silica gel (CH2Cl2/MeOH: 20/1) to provide methyl ester 36 (98 mg, 90%): 1H NMR (300 MHz, CDCl3) δ 1.61 (d, J = 6.9 Hz, 3H), 1.98–2.13 (m, 2H), 3.28 (t, J = 5.6 Hz, 1H), 3.51–3.80 (m, 3H), 3.72 (s, 3H), 3.95–3.98 (m, 2H), 4.52 (d, J = 8.0 Hz, 1H), 5.35 (q, J = 6.8 Hz, 1H), 5.77 (d, J = 7.9 Hz, 1H), 7.18 (m, 2H), 7.34 (d, J = 6.6 Hz, 1H) 7.68 (d, J = 5.6 Hz, 1H), 7.89 (s, 1H); 13C NMR (75.7 MHz, CDCl3) δ 12.8, 28.7, 29.7, 45.9, 48.7, 55.1, 62.8, 66.9, 68.4, 72.0, 104.2, 110.9, 116.2, 118.7, 120.2, 121.9, 126.4, 135.8, 136.3, 141.5, 162.2. EIMS m/e 350 (M+ 100), 333(12), 319(25), 291(20), 182(34), 168(86). HRMS calcd for C21H22N2O3: 350.1630 found 350.1629. This material was used directly in the next step.
Polyneuridine (8) (Sarpagan-16-carboxylic acid, 17-hydroxy-methyl ester, (16R)-(9CI))
The TFA (5 mL) and Et3SiH (5 mL) were added to a solution of 36 (28 mg, 0.080 mmol) in CH2Cl2 (5 mL). The reaction mixture which resulted was stirred in a sealed vessel at rt for 12 h, after which the solution was concentrated under reduced pressure. The residue was dissolved in CH2Cl2 (80 mL), and a solution of 10% aq NH4OH was added to bring the pH to 8. The organic layer was separated, washed with brine (2 × 20 mL), dried (Na2SO4), and removed under reduced pressure. The residue that resulted was purified by small column on silica gel (CH2Cl2/MeOH: 20/1) to provide 8 (25 mg, 87%): [α]D25 = 5.6 (c = 0.5, CH2Cl2) [lit. [α]D = +1 (chloroform)]71; FTIR 3267(OH), 2925, 2854, 1736(CO2Me), 1455, 1260, 1086, 799.5, 741 cm−1 (Figure 1, SI); 1H NMR (300 MHz, CDCl3) δ 1.59 (d, J = 6.8 Hz, 3H), 1.85 (ddd, 1H), 1.95(ddd, 1H), 3.01(br d, 1H), 3.12 (dd, J = 6.3 Hz, 1H), 3.18 (dd, 1H), 3.50 (m, 1H), 3.57 (m, 2H), 3.65 (d, J = 8.2 Hz, 1H), 3.73 (s, 3H), 4.18 (d, J = 9 Hz, 1H), 4.35 (d, J = 6.2 Hz, 1H), 5.23 (br q, J = 6.8 Hz, 1H), 7.10 (t, 1H), 7.16 (t, 1H), 7.34 (d, J = 7.8 Hz, 1H), 7.48 (d, J = 7.5 Hz, 1H), 8.48 (s, 1H); 13C NMR (75.7 MHz, CDCl3) δ 12.6, 21.9, 28.6, 30.4, 48.8, 52.2, 53.3, 53.6, 55.1, 63.1, 105.6, 111.1, 116.7, 118.2, 119.4, 121.7, 126.1, 134.5, 135.8, 136.1, 175.7; EIMS m/e 352(M+ 45), 336(20), 322 (100), 263(39), 249(47), 168(100), 142(82). HRMS calcd for C21H24N2O3: 352.1787 found 352.1768. The carbon NMR spectral data of 8 were in excellent agreement with those of the natural product 8 (Table 3, S.I.).
Polyneuridine Aldehyde
(6) was prepared from polyneuridine (8) in 75% yield following the procedure employed for the preparation of 35. 6: FTIR 2919, 2850, 1731(CO2Me), 1707(CHO), 1456, 1257, 1107, 747cm−1 (Figure 2, SI); EIMS m/e 350 (M+ 48), 321 (16), 260 (19), 246 (100), 231 (20), 168 (25). This material was used directly in the next step to further prove the structure of 6.
Reduction of Polyneuridine Aldehyde (6) to Polyneuridine (8)
To a solution of the above polyneuridine aldehyde (6) (2 mg, 5.7 × 10−3 mmol) and glacial acetic acid (10 uL) in methanol (1 mL) was added sodium borohydride (8.6 mg, 0.23 mmol) in small portions at 0 ° C. The mixture was stirred at this temperature for 2 h. Upon completion of the reaction, the mixture was poured into 2 mL of saturated aq sodium bicarbonate solution and extracted with methylene chloride. The extract was washed with brine, dried, and the solvent was removed under reduced pressure. The residue was purified by preparative TLC on silica gel and yield polyneuridine (8) with the same Rf as (8) prepared earlier (1.2 mg, 60 %). EIMS m/e 352 (M+ 100), 249 (31), 219 (32), 168(65), 142(82). HRMS calcd for C21H24N2O3: 352.1785 found 352.1776.
Macusine A (9)
Polyneuridine (8) (6 mg, 1.5 × 10−3mmol) was dissolved in THF (1 mL) and cooled to 0 °C. The MeI (11 mg, 0.15 mmol) was added to the above solution dropwise. The reaction mixture was stirred at 0 °C for 8 h. The solvent was removed under reduced pressure, and the residue was purified by preparative TLC on silica gel (CH2Cl2/MeOH 10/1) to provide the iodomethylated salt 23 (6.8 mg, 81 %): 1H NMR (300 MHz, MeOH-d4) δ 1.71 (d, J = 11 Hz, 3H), 2.15 (m, 1H), 2.47 (m, 1H), 3.26 (s, 3H), 3.31–3.36 (m, 2H, overlap with MeOH-d4) .3.42 (m, 2H), 3.70 (m, 2H), 3.78 (s, 3H), 4.29 (d, J = 18 Hz, 1H), 4.48 (d, J = 20.3 Hz, 1H), 4.93 (br d, partially overlap with MeOH-d4, 1H), 5.0385 (d, J = 5.91, 1H), 5.51 (q, 1H), 7,12 (t, 1H), 7.23 (t, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.57 (d, J = 8.6 Hz, 1H), 7.99 (s, NH, 1H); 13C NMR (75.7 MHz, MeOH-d4) δ 11.3, 18.6, 28.6, 29.2, 29.9, 51.8, 55.1, 59.1, 62.4, 63.9, 64.6, 101.6, 111.1, 118.2, 119.3, 119.5, 122.5, 124.8, 127.2, 130.0, 137.1, 172.7. HRMS calcd for C22H27N2O3: 367.2022 found 367.2008. This salt was treated with AgCl to give the chloride salt. The spectral data are in good agreement with the literature.76
Supplementary Material
Acknowledgement
This work was supported (in part) by NIMH and the Research Growth Initiative of the University of Wisconsin-Milwaukee.
Footnotes
Supporting Information Available: [Alternate procedure for the synthesis of 10 and 25; experimental data for 12, 32 and 33; as well as ligand screening and optimization data for copper-mediated coupling process. 1HNMR and 13C NMR spectra for the intermediates and final products as well as FT-IR for polyneuridine (8) and polyneuridine aldehyde (6).] This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Manske RHF, editor. The Alkaloids, Vols. I–XI. New York: Academic Press; 1950–1968. [Google Scholar]
- 2.The Royal Society of Chemistry, editor. Specialist Periodical Reports: The Alkaloids. London: 1970–1982. [Google Scholar]
- 3.Bentley KW. The Chemistry of Natural Products. New York: John Wiley and Sons; 1957. The alkaloids. [Google Scholar]
- 4.Pelletier SW. Chemistry of the Alkaloids. New York: Van Nostrand Reinhold Book Corporation; 1970. [Google Scholar]
- 5.Raffauf RF. A handbook of alkaloids and alkaloid-containing plants. New York: Wiley-Interscience; 1970. [Google Scholar]
- 6.Glasby JS. Encyclopedia of the alkaloids, Vols. I–III. New York: Plenum Press; 1975. [Google Scholar]
- 7.Ingham JL, Koskinen A, Lounasmaa M. Progress in the Chemistry of Organic Natural Products. New York: Springer-Verlag; 1983. [Google Scholar]
- 8.Keawpradub N, Eno-Amooquaye E, Burke PJ, Houghton P. J. Planta Med. 1999;65:311. doi: 10.1055/s-1999-13992. [DOI] [PubMed] [Google Scholar]
- 9.Keawpradub N, Kirby GC, Steele JC, Houghton P. J. Planta Med. 1999;65:690. doi: 10.1055/s-1999-14043. [DOI] [PubMed] [Google Scholar]
- 10.Burke DE, Cook JM, Le Quesne PW. J. Am. Chem. Soc. 1973;95:546. [Google Scholar]
- 11.Siddiqui S, Siddiqui RHJ. Indian Chem. Soc. 1931;8:667. [Google Scholar]
- 12.Chen C, Lieberman DR, Larsen RD, Reamer RA, Verhoeven TR, Reider PJ. Tetrahedron lett. 1994;35:6981. [Google Scholar]
- 13.Brugada J, Brugada P. Am. J. Cardiol. 1996;78:69. doi: 10.1016/s0002-9149(96)00505-x. [DOI] [PubMed] [Google Scholar]
- 14.Slowinski S, Rajch D, Zabowka M. Przegl. Lek. 1996;53:196. [PubMed] [Google Scholar]
- 15.Mozo dRF, Moreno J, Bodegas A, Melchor JC, Fernandez LL, Aranguren G. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994;56:63. doi: 10.1016/0028-2243(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 16.Creasey WA. The Monoterpenoid Indole Alkaloids. In: Saxton JE, editor. Heterocyclic Compounds; Indole Series. Vol. 25. New York: John Wiley and Sons; 1983. p. 783. [Google Scholar]
- 17.Mest HJ, Winkler J, Foerster W. Acta Biol. Med. Ger. 1977;36:1193. [PubMed] [Google Scholar]
- 18.Bi Y, Hamaker LK, Cook JM. The Synthesis of Macroline Related Alkaloids. In: Rahman ABF, editor. Studies in Natural Products Chemistry: Bioactive Natural Products. Vol. 13. Amsterdam: Elsevier Science; 1993. p. 383. [Google Scholar]
- 19.Hamaker LK, Cook JM. The synthesis of macroline related sarpagine alkaloids. Alkaloids: Chemical and Biological Perspectives. 1995;Vol. 9:23. [Google Scholar]
- 20.Benthe HF. Naunyn-Schmiedebergs Arch. Exptl. Pathol. Pharmakol. 1956;229:82. [PubMed] [Google Scholar]
- 21.Ruppert M, Ma X, Stoeckigt J. Curr. Org. Chem. 2005;9:1431. [Google Scholar]
- 22.Koskinen A, Lounasmaa M. Planta Med. 1982;45:248. doi: 10.1055/s-2007-971385. [DOI] [PubMed] [Google Scholar]
- 23.Pfitzner A, Stöckigt J. Tetrahedron Lett. 1983;24:5197. [Google Scholar]
- 24.Yang L, Hill M, Wang M, Panjikar S, Stoeckigt J. Angew. Chem. Int. Ed. 2009;48:5211. doi: 10.1002/anie.200900150. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Wearing XZ, Cook JM. J. Am. Chem. Soc. 2004;126:1358. doi: 10.1021/ja039798n. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Wearing XZ, Cook JM. J. Org. Chem. 2005;70:3963. doi: 10.1021/jo040282b. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Martin SF. 214th National Meeting of the American Chemical Society; September 9–11; Las Vegas, NV. ORGN 204. [Google Scholar]
- 28.Woodward RB. Angew. Chem. 1956;68:13. [Google Scholar]
- 29.Bartlett MF, Lambert BF, Werblood HM, Taylor WI. J. Am. Chem. Soc. 1963;85:475. [Google Scholar]
- 30.Dogru E, Warzecha H, Seibel F, Haebel S, Lottspeich F, Stoeckigt J. Eur. J. Biochem. 2000;267:1397. doi: 10.1046/j.1432-1327.2000.01136.x. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Liao X, Cook JM. Org. Lett. 2002;4:4681. doi: 10.1021/ol020209c. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Cook JM. Org. Lett. 2000;2:2057. doi: 10.1021/ol000095+. [DOI] [PubMed] [Google Scholar]
- 33.Yu J, Wang T, Wearing XZ, Ma J, Cook JM. J. Org. Chem. 2002;68:5852. doi: 10.1021/jo030116o. [DOI] [PubMed] [Google Scholar]
- 34.Yu P, Wang T, Yu F, Cook JM. Tetrahedron Lett. 1997;38:6819. [Google Scholar]
- 35.Herrmann WA, Elison M, Fischer J, Köcher C, Artus GRJ. Angew. Chem. Int. Ed. 1995;34:2371. [Google Scholar]
- 36.Liu X, Wang T, Xu Q, Ma C, Cook JM. Tetrahedron Lett. 2000;41:6299. [Google Scholar]
- 37.Bi Y, Cook JM. Tetrahedron Lett. 1993;34:4501. [Google Scholar]
- 38.Magnus P, Mugrae B, DeLuca M, Cain GA. J. Am. Chem. Soc. 1989;111:786. [Google Scholar]
- 39.Magnus P, Mugrae B, DeLuca M, Cain GA. J. Am. Chem. Soc. 1990;112:5220. [Google Scholar]
- 40.Li J, Wang T, Yu P, Peterson A, Weber R, Soerens D, Grubisha D, Bennett D, Cook JM. J. Am. Chem. Soc. 1999;121:6998. [Google Scholar]
- 41.Sarma PVVS, Cook JM. Org. Lett. 2006;8:1017. doi: 10.1021/ol0526266. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett MF, Sklar R, Taylor WI, Schlittler E, Amai RLS, Beak P, Bringi NV, Wenkert E. J. Am. Chem. Soc. 1962;84:622. [Google Scholar]
- 43.Mancuso AJ, Huang SL, Swern DJ. J. Org. Chem. 1978;43:2480. [Google Scholar]
- 44.Dess DB, Martin JC. J. Am. Chem. Soc. 1991;113:7277. [Google Scholar]
- 45.Nicolaou KC, Baran PS, Zhong Y. J. Am. Chem. Soc. 2001;123:3183. doi: 10.1021/ja004218x. [DOI] [PubMed] [Google Scholar]
- 46.Wan ASC, Yokota M, Ogata K, Aimi N, Sakai S. Heterocycles. 1987;26:1211. [Google Scholar]
- 47.Corey EJ, Kim CU. J. Am. Chem. Soc. 1972;94:7586. [Google Scholar]
- 48.Banerji J, Das B, Chakrabarti R, Shoolery JN. Ind. J.Chem. (Section B) 1987;26B:709. [Google Scholar]
- 49.Pfitzner A, Stoeckigt J. J. Chem. Soc., Chem. Comm. 1983;459 [Google Scholar]
- 50.Barton DHR, Brewster AG, Hui RAHF, Lester DJ, Ley SV, Back TG. J. Chem. Soc., Chem. Commun. 1978;952 [Google Scholar]
- 51.Solé D, Peidró E, Bonjoch J. Org. Lett. 2000;2:2225. doi: 10.1021/ol005973i. [DOI] [PubMed] [Google Scholar]
- 52.Solé D, Urbaneja X, Bonjoch J. Adv. Synth. Catal. 2004;346:1646. [Google Scholar]
- 53.Wang T, Cook JM. 220th ACS National Meeting; 2000, August 20–24;; Washington, DC, United States. ORGN-140. [Google Scholar]
- 54.Yin W, Ma j, Rivas FM, Cook JM. Org. Lett. 2007;9:295. doi: 10.1021/ol062762q. [DOI] [PubMed] [Google Scholar]
- 55.Kabir MS, Van Linn ML, Monte A, Cook JM. Org. Lett. 2008;10:3363. doi: 10.1021/ol801149n. [DOI] [PubMed] [Google Scholar]
- 56.Amatore C, Carré E, Jutand A, M'Barki MA. Organometallics. 1995;14:1818. [Google Scholar]
- 57.Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 58.Liu X. Ph.D Thesis. University of Wisconsin-Milwaukee; 2002. [Google Scholar]
- 59.Liao X, Zhou H, Yu J, Cook JM. J. Org. Chem. 2006;71:8884. doi: 10.1021/jo061652u. [DOI] [PubMed] [Google Scholar]
- 60.Cao H, Yu J, Wearing X, Zhang C, Liu X, Cook JM. Tetrahedron Lett. 2003;44:8013. [Google Scholar]
- 61.Ensley HE, Buescher RR, Lee K. J. Org. Chem. 1982;47:404. [Google Scholar]
- 62.Yang J, Rallapalli SK, Cook JM. Tetrahedron Lett. 2010;51:815. [Google Scholar]
- 63.Krafft ME, Cran JW. Synlett. 2005;8:1263. [Google Scholar]
- 64.Wang T. Ph.D. Thesis. University of Wisconsin-Milwaukee; 2001. [Google Scholar]
- 65.Fuji K. Chem. Rev. 1993;93:2037. [Google Scholar]
- 66.Wang R, Yang C-W. Huaxue(Chinese) 1996;54:169. [Google Scholar]
- 67.Corey EJ, Guzman-Perez A. Angew. Chem. Int. Ed. 1998;37:388. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 68.Zhou H, Liao X, Yin W, Ma J, Cook JM. J. Org. Chem. 2006;71:251. doi: 10.1021/jo052081t. [DOI] [PubMed] [Google Scholar]
- 69.Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994;639 [Google Scholar]
- 70.Yamada S, Morizono D, Yamamoto K. Tetrahedron Letters. 1992;33:4329. [Google Scholar]
- 71.Antonaccio LD, Pereira NA, Gilbert B, Vorbrueggen H, Budzikiewicz H, Wilson JM, Durham LJ, Djerassi CJ. Am. Chem. Soc. 1962;84:2161. [Google Scholar]
- 72.Jokela R, Lounasmaa M. Heterocycles. 1996;43:1015. [Google Scholar]
- 73.Yu J, Wang T, Liu X, Deschamps J, Flippen-Anderson J, Liao X, Cook JM. J. Org. Chem. 2003;68:7565. doi: 10.1021/jo030006h. [DOI] [PubMed] [Google Scholar]
- 74.Loh T-P, Cao G-Q, Pei J. Tetrahedron Lett. 1998;39:1453. [Google Scholar]
- 75.Kuehne ME, Wang T, Seraphin D. J. Org. Chem. 1996;61:7873. doi: 10.1021/jo961023s. [DOI] [PubMed] [Google Scholar]
- 76.McPhail AT, Robertson JM, Sim GA. J. Chem. Soc. 1963;1832 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.