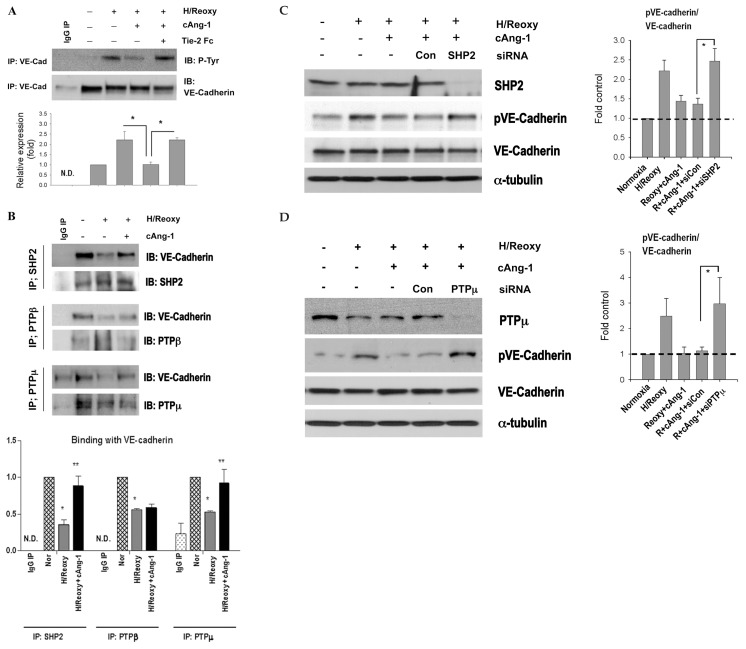
Figure 2.
cAng-1 reduces phosphorylation of VE-cadherin by interaction with phosphatases, such as SHP2 or PTPμ in HUVECs. (A) Immunoprecipitation with anti–VE-cadherin antibody followed by immunoblotting with phospho-tyrosine (P-Tyr) antibody. The same filter was reprobed with anti–VE-cadherin antibody, demonstrating equal loading. pY-VE-cadherin was increased after H/Reoxy. cAng-1 markedly reduced pY-VE-cadherin, which was reversed by Tie-2 Fc (n = 3, *P < 0.05; N.D., not determined). (B) Immunoprecipitates by anti-SHP2, anti-PTPβ or anti-PTPμ antibody were immunoblotted with anti–VE-cadherin antibody. Binding of VE-cadherin with phosphatase significantly decreased in H/Reoxy conditions. cAng-1 significantly restored the binding of VE-cadherin with SHP2 or PTPμ but negligibly with PTPβ (n = 3, *P < 0.05 versus Nor; **P < 0.05 versus Hy; N.D., not determined). (C, D) Inhibition of phosphatase using SHP2 siRNA or PTPμ siRNA remarkably increased pVE-cadherin (Tyr-658) levels (C, D, left) resulting in the increased ratio of phospho-VE-cadherin to VE-cadherin (C, D, right) (n = 3, *P < 0.05).