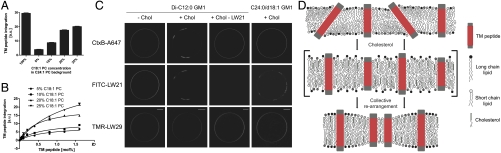
Fig. 4.
Hydrophobic mismatch and cholesterol determine lateral lipid distribution. (A) Trp fluorescence emission indicates integration of LW21 peptide into LUVs with 100, 0, 10, 20, and 25 mol% C18∶1 PC in a C24∶1 PC background containing 30 mol% cholesterol (bars = SD, n = 3). (B) Trp fluorescence emission indicates integration of the titrated LW21 peptide into LUVs with 5, 10, 20, and 25 mol% C18∶1 PC in a C24∶1 PC background containing 30 mol% cholesterol (bars = SD, n = 3). (C) Fluorescence microscopy images of GUVs depicting the localization of FITC-LW21 (1.5 mol%), TMR-LW29 (1.5 mol%), and CtxB-A647-labeled Di-C12∶0 GM1 (0.1 mol%) before and after MBCD-cholesterol loading and in the absence of FITC-LW21. Last column shows CtxB-A647-labeled C24∶0/dC18∶1 GM1 (0.1 mol%) after MBCD-cholesterol loading. bar = 10 μm. (D) Scheme of cholesterol’s function to rearrange TM peptide and lipid distribution according to hydrophobic length. It extends the acyl chains of the lipids making them less adaptable to mismatching TM peptides.