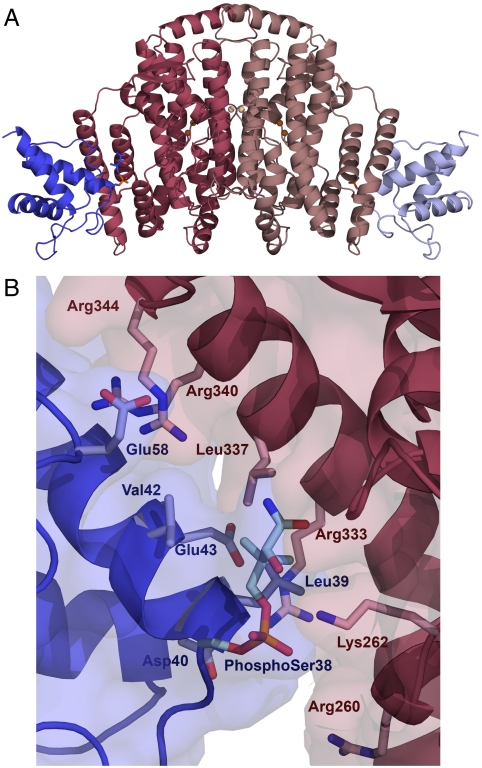
Fig. 1.
The desaturase–ACP complex structure. (A) Fully occupied complex of crystal form 2 in cartoon representation. The desaturase monomers are colored in dark and light pink, and the ACP in dark and light blue. The defined portions of the phospho-serine and pantetheine linkage are depicted as stick representations, and the catalytic irons are shown as brown spheres with the metal ions on the dimer interface in light brown. (B) The desaturase–ACP interface in crystal form 2, with the desaturase chain B (pink) and the ACP chain C (blue). Residues participating in the desaturase–ACP interaction are shown in stick representation, with the phospho-serine38 and the defined part of the pantetheine chain depicted in pale blue.