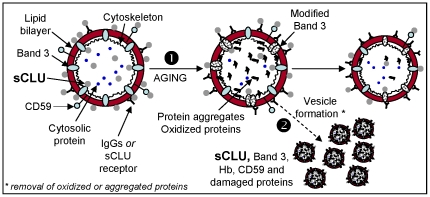
Figure 5. Proposed model of sCLU involvement in RBCs plasma membrane integrity, cellular senescence and vesiculation.
Erythrocytic sCLU localizes at both sides of the plasma membrane in association with non-cytoskeletal areas, as well as in the cytosol (see also, Antonelou et al., accompanying paper). At the intracellular side of the RBCs membrane sCLU may bind Band 3, Hb and/or other cytoskeleton-free membrane portions. On the other hand, the sCLU that localizes at the extracellular side of the RBCs membrane can attach to membrane by binding to Band 3, CD59, plasma membrane IgGs or to an currently unknown sCLU-specific receptor. Physiological in vivo or ex vivo RBCs senescence (1) is associated with cytosol, cytoskeleton and membrane structural alterations, including Band 3 modifications, increased membrane binding of IgGs, proteolysis, protein aggregation and increased oxidation defects. Vesiculation (2), a self-protective mechanism of mammalian erythrocytes, removes oxidized proteins and aggregates from both plasma membrane and cytosol thereby postponing the untimely elimination of otherwise healthy erythrocytes. This process takes place through the entire in vivo or ex vivo lifespan of RBCs and is functionally connected to the release of sCLU-, Band 3-, CD59-, Hb- and IgGs-containing vesicles. We propose that vesicular sCLU by following its membrane linkers (e.g. Band 3) or other unknown cytosolic interacting proteins assists via its chaperone function in the disposal of non-functional or death signalling effective material from RBCs.