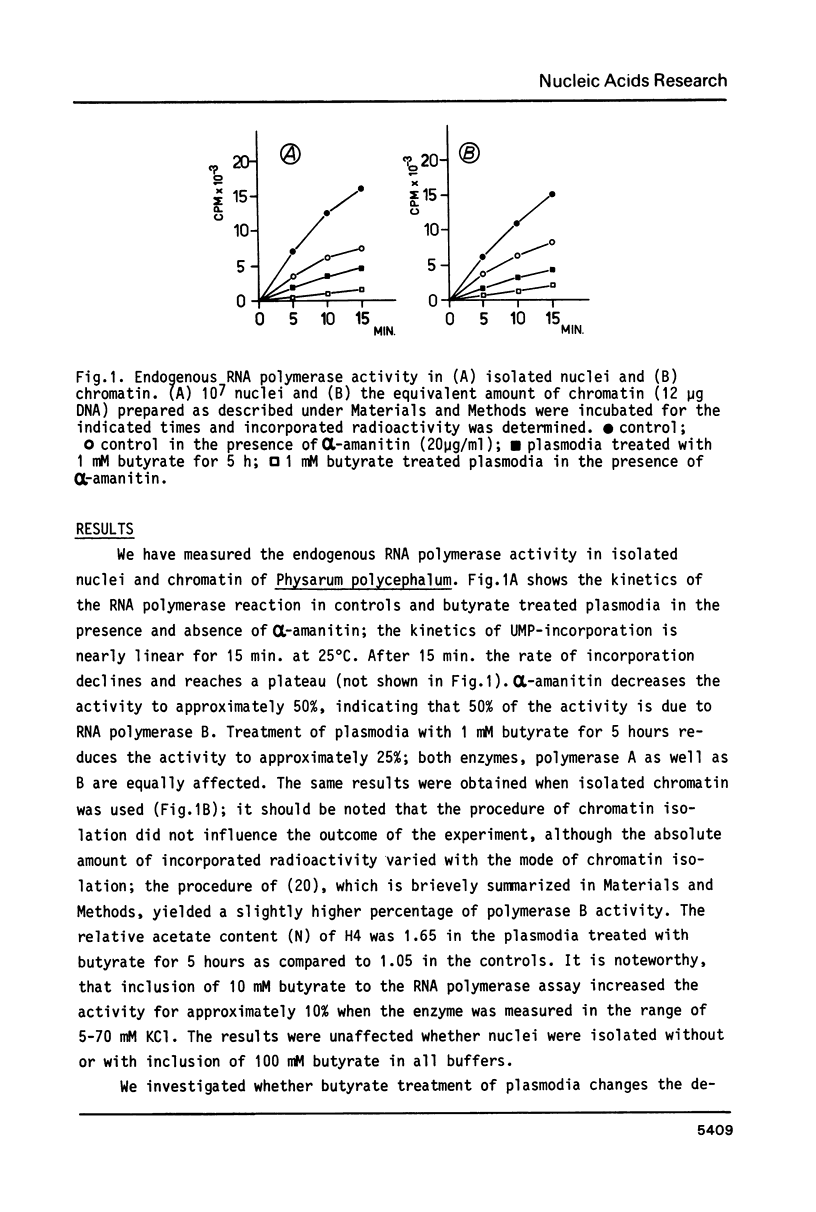
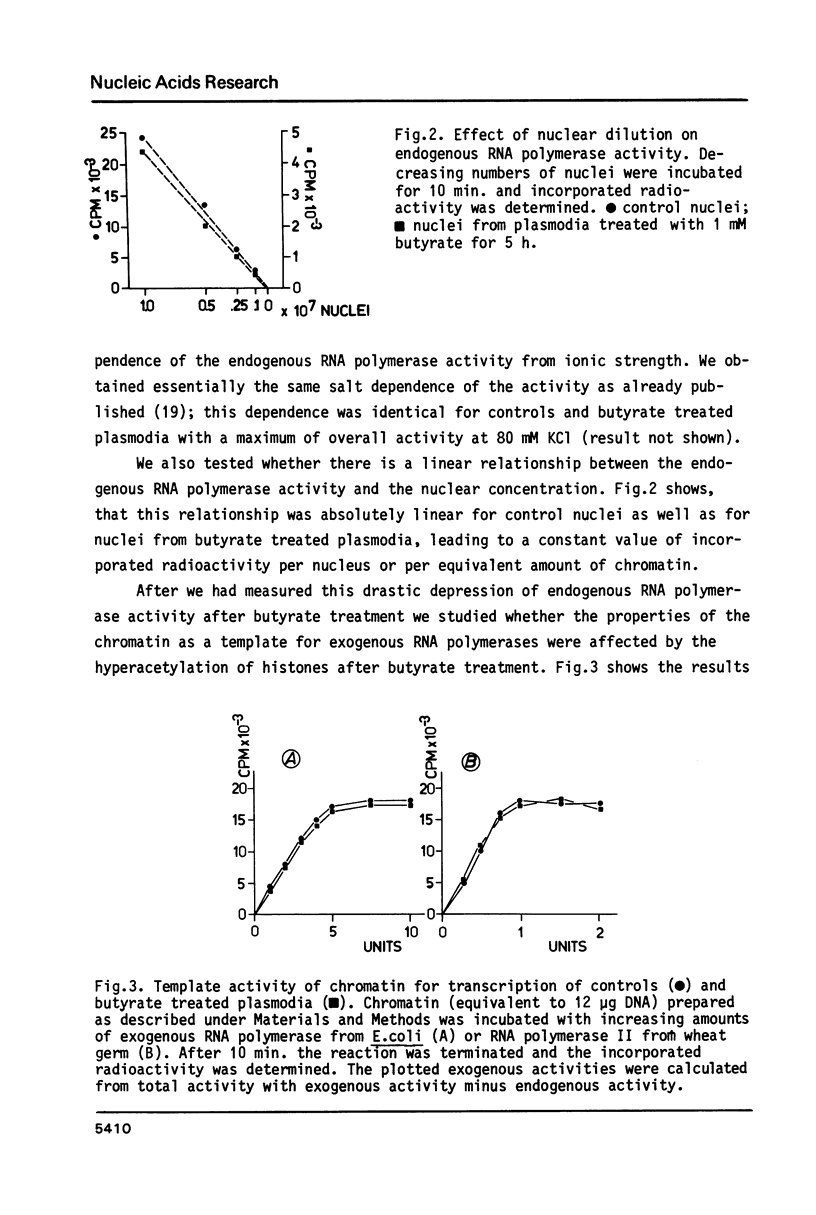
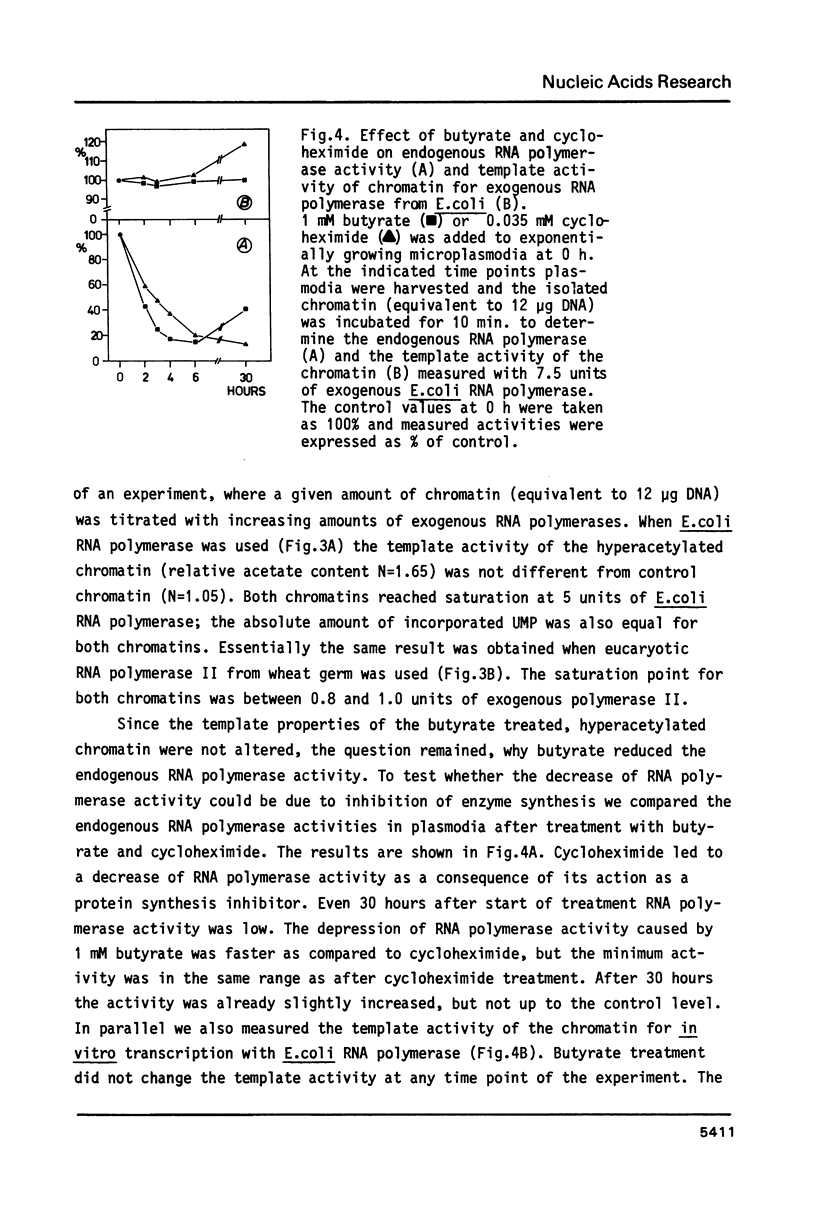
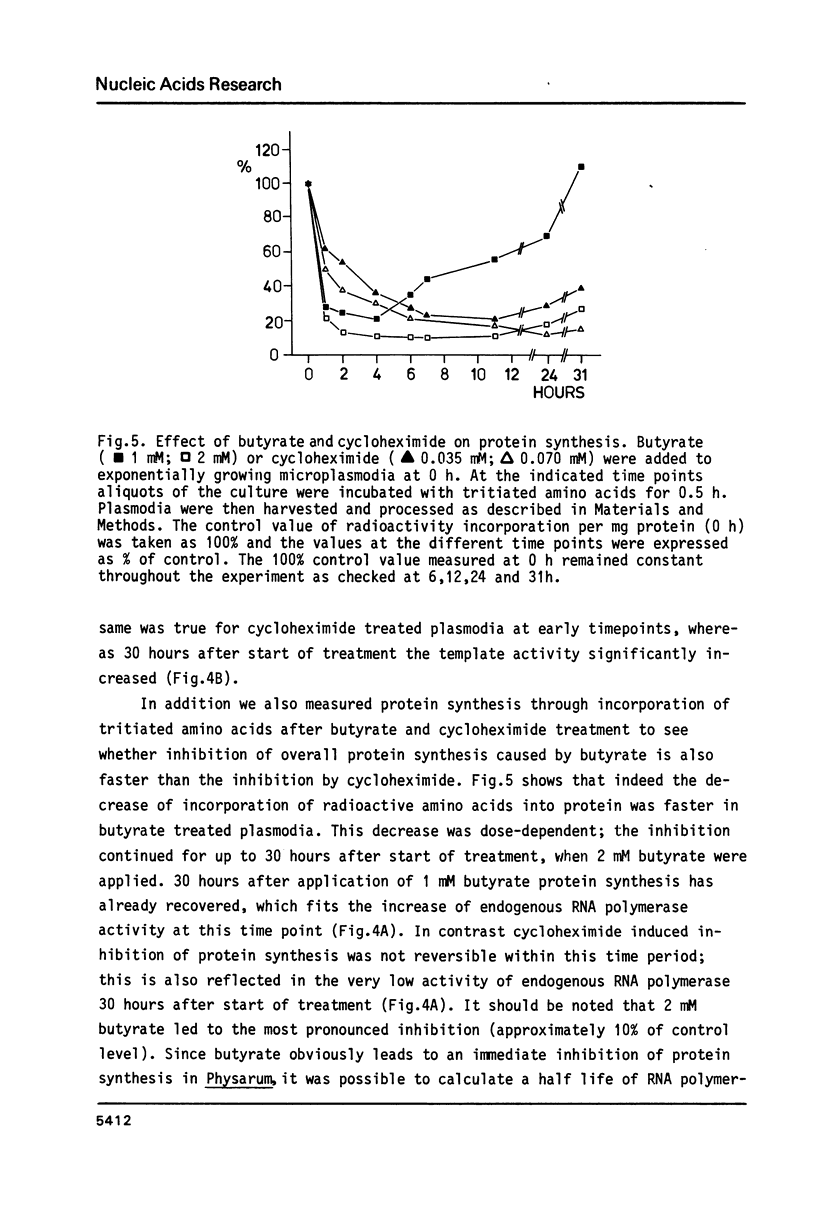
Abstract
We have studied the effect of sodium-n-butyrate on endogenous RNA polymerase in Physarum polycephalum. 1 mM butyrate strongly reduces RNA polymerase activity measured in isolated nuclei or chromatin; both RNA polymerase A as well as the alpha-amanitin sensitive RNA polymerase B are equally affected. Despite a concomitant hyperacetylation of histone H4 the template activity of chromatin, as analyzed by in vitro transcription of the chromatin with exogenous RNA polymerase from E. coli or RNA polymerase II from wheat germ, remains unaltered as compared to untreated control chromatin, indicating that there is no positive correlation between histone acetylation and template activity of chromatin for transcription in this organism. The results further indicate, that butyrate acts primarily as a quick but reversible inhibitor of protein synthesis in Physarum; the fast decrease of endogenous RNA polymerase activity after butyrate treatment is due to inhibition of enzyme synthesis rather than inactivation of other factors necessary for transcription.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S., Gunther H., Morisi A. Butyrate reversibly arrests the proliferation of normal and Rous sarcoma virus-infected chicken heart mesenchymal cells. Life Sci. 1984 Feb 20;34(8):803–808. doi: 10.1016/0024-3205(84)90388-6. [DOI] [PubMed] [Google Scholar]
- Bell P. A., Jones C. N. Cytotoxic effects of butyrate and other 'differentiation inducers' on immature lymphoid cells. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1202–1208. doi: 10.1016/0006-291x(82)91378-x. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Gruss R. J., Allfrey V. G. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem. 1981 Sep 25;256(18):9612–9621. [PubMed] [Google Scholar]
- Chahal S. S., Matthews H. R., Bradbury E. M. Acetylation of histone H4 and its role in chromatin structure and function. Nature. 1980 Sep 4;287(5777):76–79. doi: 10.1038/287076a0. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev Biol. 1982 Oct;93(2):404–415. doi: 10.1016/0012-1606(82)90127-0. [DOI] [PubMed] [Google Scholar]
- Covault J., Perry M., Chalkley R. Effects of histone hyperacetylation and hypoacetylation on RNA synthesis in HTC cells. J Biol Chem. 1982 Nov 25;257(22):13433–13440. [PubMed] [Google Scholar]
- Csordas A., Multhaup I., Grunicke H. Transcription of chemically acetylated chromatin with homologous RNA polymerase B. Biosci Rep. 1984 Feb;4(2):155–163. doi: 10.1007/BF01120312. [DOI] [PubMed] [Google Scholar]
- Davies K. E., Walker I. O. In vitro transcription of RNA in nuclei, nucleoli and chromatin from physarum polycephalum. J Cell Sci. 1977 Aug;26:267–279. doi: 10.1242/jcs.26.1.267. [DOI] [PubMed] [Google Scholar]
- Dobson M. E., Ingram V. M. In vitro transcription of chromatin containing histones hyperacetylated in vivo. Nucleic Acids Res. 1980 Sep 25;8(18):4201–4219. doi: 10.1093/nar/8.18.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti V., Russo E., de Cristini F., Graziosi G., Micali F., Crane-Robinson C. Histone modification in early and late Drosophila embryos. Biochem J. 1984 Mar 1;218(2):321–329. doi: 10.1042/bj2180321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Pleger G. L., Keevert J. B., Johmann C. A. Studies on histone fraction F2A1 in macro- and micronuclei of Tetrahymena pyriformis. J Cell Biol. 1973 Jun;57(3):773–781. doi: 10.1083/jcb.57.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröbner P., Loidl P. Response of the dTMP-synthesizing enzymes to differentiation processes in Physarum polycephalum. Exp Cell Res. 1983 Apr 1;144(2):385–391. doi: 10.1016/0014-4827(83)90418-4. [DOI] [PubMed] [Google Scholar]
- Karpov V. L., Preobrazhenskaya O. V., Mirzabekov A. D. Chromatin structure of hsp 70 genes, activated by heat shock: selective removal of histones from the coding region and their absence from the 5' region. Cell. 1984 Feb;36(2):423–431. doi: 10.1016/0092-8674(84)90235-6. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilley D. M., Berendt A. R. The gross level of in vitro RNA synthesis in HeLa nuclei is unaltered by histone hyperacetylation. Biochem Biophys Res Commun. 1979 Oct 12;90(3):917–924. doi: 10.1016/0006-291x(79)91915-6. [DOI] [PubMed] [Google Scholar]
- Lohr D., Hereford L. Yeast chromatin is uniformly digested by DNase-I. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4285–4288. doi: 10.1073/pnas.76.9.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P., Gröbner P., Csordas A., Puschendorf B. Cell-cycle-dependent effects of sodium-n-butyrate in Physarum polycephalum. J Cell Sci. 1982 Dec;58:303–311. doi: 10.1242/jcs.58.1.303. [DOI] [PubMed] [Google Scholar]
- Loidl P., Loidl A., Puschendorf B., Gröbner P. Lack of correlation between histone H4 acetylation and transcription during the Physarum cell cycle. 1983 Sep 29-Oct 5Nature. 305(5933):446–448. doi: 10.1038/305446a0. [DOI] [PubMed] [Google Scholar]
- Marushige K. Activation of chromatin by acetylation of histone side chains. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3937–3941. doi: 10.1073/pnas.73.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Oudet P., Wasylyk B., Chambon P. Effect of histone acetylation on structure and in vitro transcription of chromatin. Nucleic Acids Res. 1978 Oct;5(10):3523–3547. doi: 10.1093/nar/5.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Nickol J. M., Felsenfeld G., Rau D. C. Histone hyperacetylation has little effect on the higher order folding of chromatin. Nucleic Acids Res. 1983 Jun 25;11(12):4065–4075. doi: 10.1093/nar/11.12.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res. 1971 Jun;66(2):305–316. doi: 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation of the nuclear histones from the Myxomycete, Physarum polycephalum. Arch Biochem Biophys. 1969 Nov;134(2):577–589. doi: 10.1016/0003-9861(69)90320-8. [DOI] [PubMed] [Google Scholar]
- Muller S., Erard M., Burggraf E., Couppez M., Sautière P., Champagne M., Van Regenmortel M. H. Immunochemical detection of changes in chromatin subunits induced by histone H4 acetylation. EMBO J. 1982;1(8):939–944. doi: 10.1002/j.1460-2075.1982.tb01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. A., Perry M., Sealy L., Chalkley R. DNAse I preferentially digests chromatin containing hyperacetylated histones. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1346–1353. doi: 10.1016/0006-291x(78)90337-6. [DOI] [PubMed] [Google Scholar]
- Oberhauser H., Csordas A., Puschendorf B., Grunicke H. Increase in initiation sites for chromatin directed RNA synthesis by acetylation of chromosomal proteins. Biochem Biophys Res Commun. 1978 Sep 14;84(1):110–116. doi: 10.1016/0006-291x(78)90270-x. [DOI] [PubMed] [Google Scholar]
- Oliva R., Mezquita C. Histone H4 hyperacetylation and rapid turnover of its acetyl groups in transcriptionally inactive rooster testis spermatids. Nucleic Acids Res. 1982 Dec 20;10(24):8049–8059. doi: 10.1093/nar/10.24.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Littau V. C., Allfrey V. G. Changes in histone acetyl content and in nuclear non-histone protein composition of avian erythroid cells at different stages of maturation. J Biol Chem. 1974 Nov 25;249(22):7358–7368. [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Stevens M. S., Aliabadi Z., Moore M. R. Associated effects of sodium butyrate on histone acetylation and estrogen receptor in the human breast cancer cell line MCF-7. Biochem Biophys Res Commun. 1984 Feb 29;119(1):132–138. doi: 10.1016/0006-291x(84)91628-0. [DOI] [PubMed] [Google Scholar]
- Takaku F., Nakao K., Ono T., Terayama H. Changes in histone acetylation and RNA synthesis in the spleen of polycythemic mouse after erythropoietin injection. Biochim Biophys Acta. 1969 Dec 16;195(2):396–400. doi: 10.1016/0005-2787(69)90646-7. [DOI] [PubMed] [Google Scholar]
- Turnock G. Patterns of nucleic acid synthesis in Physarum polycephalum. Prog Nucleic Acid Res Mol Biol. 1979;23:53–104. doi: 10.1016/s0079-6603(08)60131-2. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangh L., Ruiz-Carrillo A., Allfrey V. G. Separation and analysis of histone subfractions differing in their degree of acetylation: some correlations with genetic activity in development. Arch Biochem Biophys. 1972 May;150(1):44–56. doi: 10.1016/0003-9861(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Xue S., Rao P. N. Sodium butyrate blocks HeLa cells preferentially in early G1 phase of the cell cycle. J Cell Sci. 1981 Oct;51:163–171. doi: 10.1242/jcs.51.1.163. [DOI] [PubMed] [Google Scholar]
- Yukioka M., Sasaki S., Henmi S., Matsuo M., Hatayama T., Inoue A. Transcribing chromatin is not preferentially enriched with acetylated histones. FEBS Lett. 1983 Jul 25;158(2):281–284. doi: 10.1016/0014-5793(83)80595-x. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]


