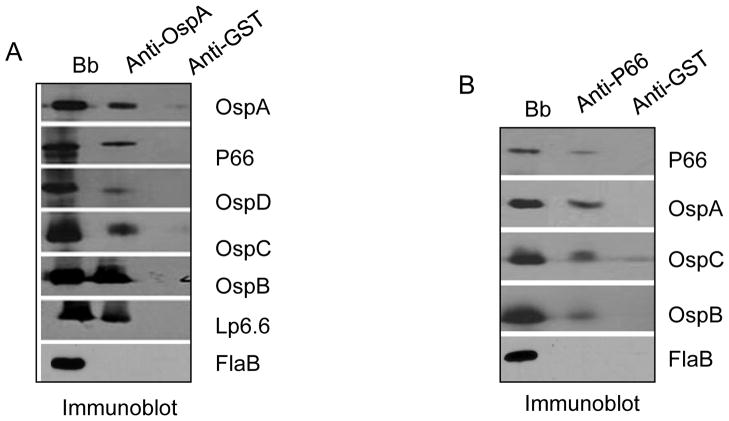
Figure 3. Interaction of subunits assessed by protein cross-linking/co-immunoprecipitation.
B. burgdorferi (Bb) lysates were treated with a protein cross-linker (DSP) and immunoprecipitated either with OspA (panel A) or P66 (panel B) antibodies. Antibodies against glutathione-S-transferase (GST) were used as a control. Left lane (Bb) donates antibody recognition of target proteins in B. burgdorferi lysates without the immunoprecipitation step. Immunoprecipitated proteins (middle and right lanes) were identified by antibodies against OspA, P66, OspD, -C, -B and Lp6.6. As an additional control, immunoprecipitates were also probed with antibodies against the B. burgdorferi protein (FlaB), which was not expected to be present in the complex.