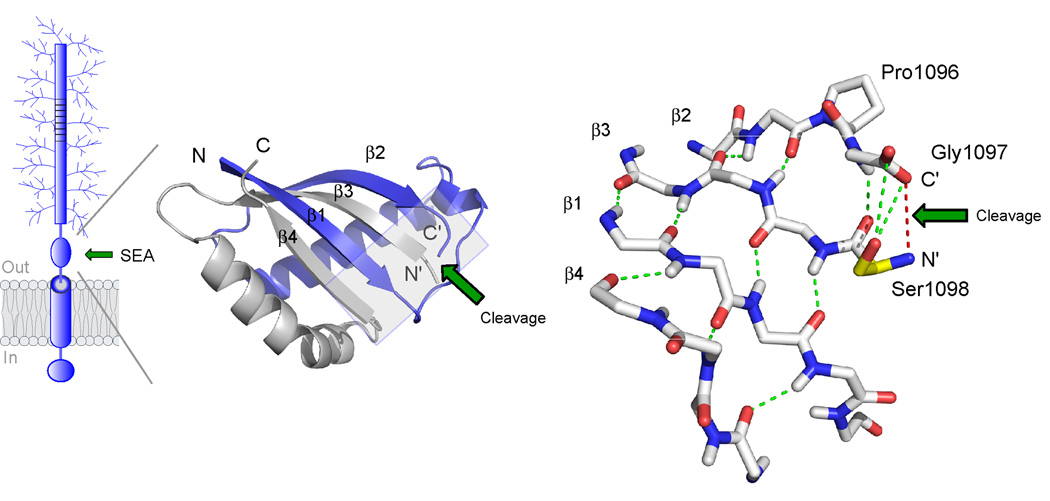
Figure 3. Structure of the self-cleaving SEA domain of MUC1.
The structure is based on NMR spectroscopy data ([27], PDB number 2ACM). At left is shown the folding topology and secondary structure of the cleaved SEA heterodimer with the two intertwined subunits colored in blue and grey, respectively. Autoproteolytic cleavage occurs at the edge of a four-stranded β-sheet to generate novel N’ and C’ peptide termini, as indicated. The structure on the right shows a detailed view of the peptide backbone at the cleavage site (boxed area in figure to the left). Autoproteolytic cleavage has occurred between glycine and serine in the tight turn Pro1096-Gly1097-Ser1098 as a result of conformational strain generated by the β-sheet structure (hydrogen bonds in green) and the nucleophilic action of the serine hydroxyl (Ser1098 side chain in yellow). The location of the former glycine-serine peptide bond is illustrated by a red line. The site of cleavage is denoted by a green arrow.