Figure 4.
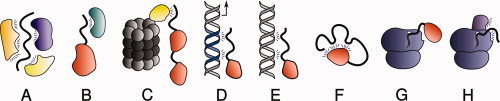
Mechanisms preventing disordered proteins from degradation by default. Proteins can be stabilized when involved in intermolecular interactions with (A) other members of the same stable complex (e.g., the cytoplasmic domain of neuroligin 3 with S-SCAM and PSD95); (B) a nanny protein (e.g., p53 with Hdmx); (C) a proteasome gatekeeper (e.g., p53 with NQO1); (C) a nanny protein (e.g., p53 with Hdmx); (D) a functional DNA binding site; (E) a nonfunctional “decoy” DNA binding site. F: Proteins can also be protected by intramolecular interactions (e.g., calcinin N). The nascent chain of an intrinsically disordered protein can be protected from degradation by (G) local folding, by interaction with a partner or (H) interacting with the ribosome or with ribosome-associated proteins.
