Figure 15.
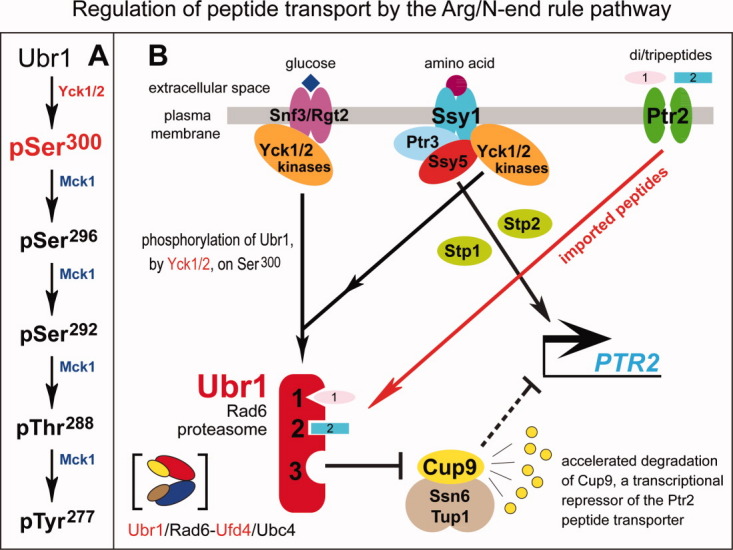
Regulation of peptide import by the Arg/N-end rule pathway in S. cerevisiae, and inputs by the amino acid-sensing SPS pathway. A: The “primed” cascade of Ubr1 phosphorylation in which the Yck1/Yck2-mediated phosphorylation on Ser300 of Ubr1 is essential for the normal regulation of peptide import39 [see also the legend to Fig. 7(A,B)]. B: Ubr1-mediated regulation of peptide import, and the involvement of the SPS pathway.27,29,138,295,296 Cup9 is a transcriptional repressor of the regulon that includes PTR2, which encodes the major importer of di/tripeptides. In the absence of Ubr1 (in ubr1Δ cells), Cup9 becomes long-lived, accumulates to high levels, and extinguishes expression of Ptr2. Therefore, ubr1Δ cells cannot import di/tripeptides. In wild-type (UBR1) cells growing in the absence of extracellular di/tripeptides, a relatively low but nonzero number of Ubr1 molecules have their third substrate-binding site “open” (not autoinhibited) and therefore can target Cup9 for degradation (t1/2 ∼ 5 min) via its internal degron, resulting in a low but significant steady-state concentration of Cup9 and, thus, a weak but significant expression of the Ptr2 transporter. In wild-type (UBR1) cells growing in the presence of extracellular di/tripeptides (some of which bear Type-1 and Type-2 destabilizing N-terminal residues), the imported peptides interact with the Type-1 and Type-2 binding sites of Ubr1. This binding allosterically increases the fraction of Ubr1 molecules whose third (Cup9-specific) site is “open” (active). The resulting decrease in the half-life of Cup9 (from ∼5 min to below 1 min) results in a low concentration of Cup9, and consequently to a strong induction of the Ptr2 transporter.27,29,138 Also shown is the amino acid-sensing SPS pathway (see Functions of the N-end rule pathway vis-á-vis their mechanisms section for details and additional references), which can influence the import of peptides at least in part through the Yck1/Yck2-mediated phosphorylation of Ubr1 on Ser.300 This phosphorylation is required (through a mechanism that remains to be determined) for normal levels of Ubr1 activity in the Ptr2-Cup9-Ubr1 circuit.39 [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
